Abstract
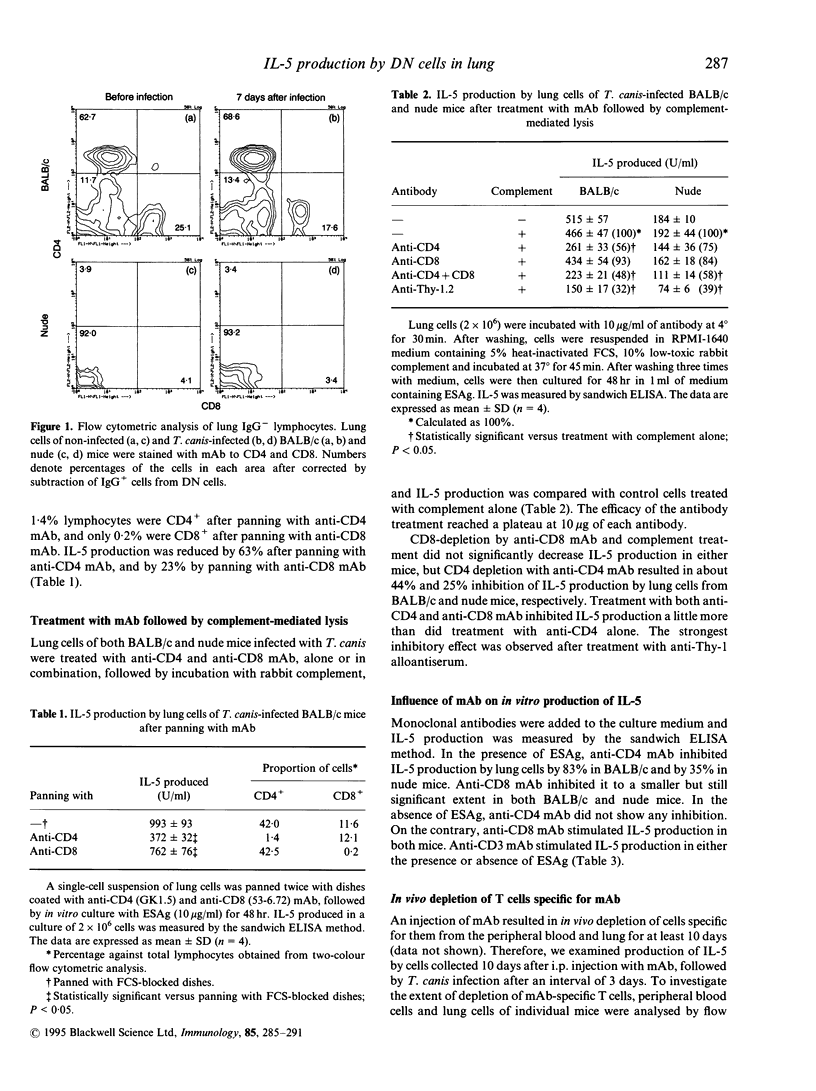
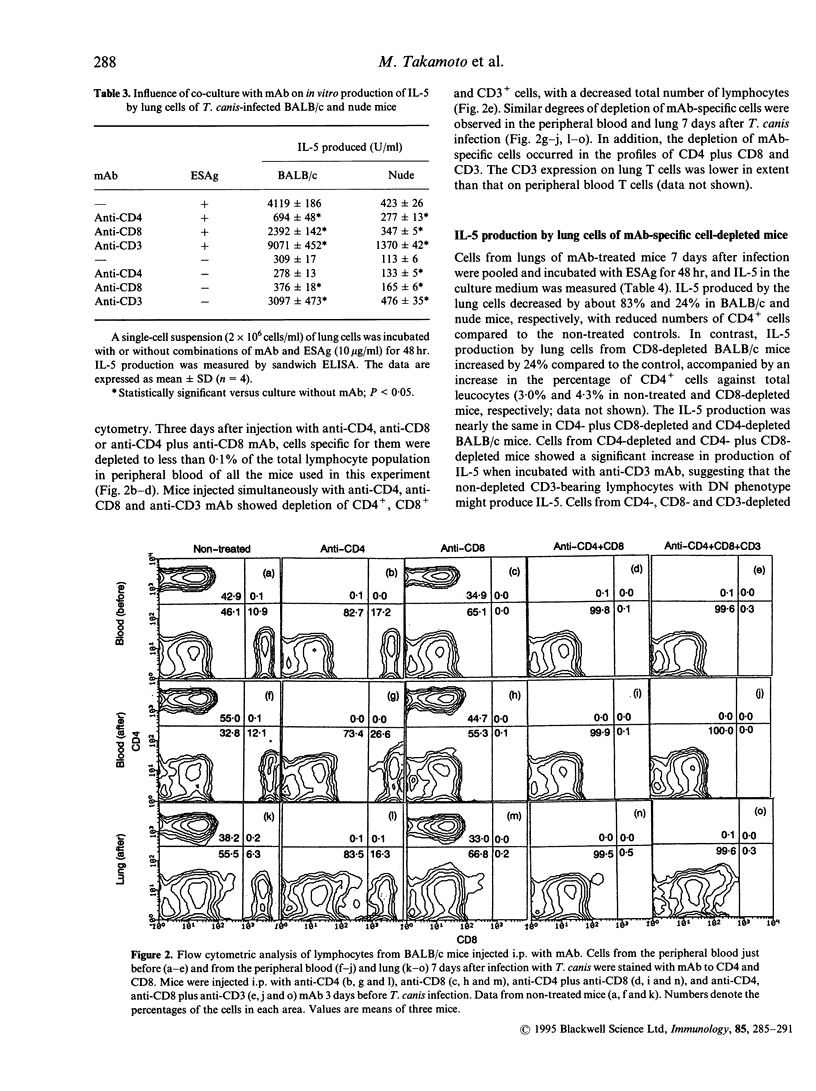
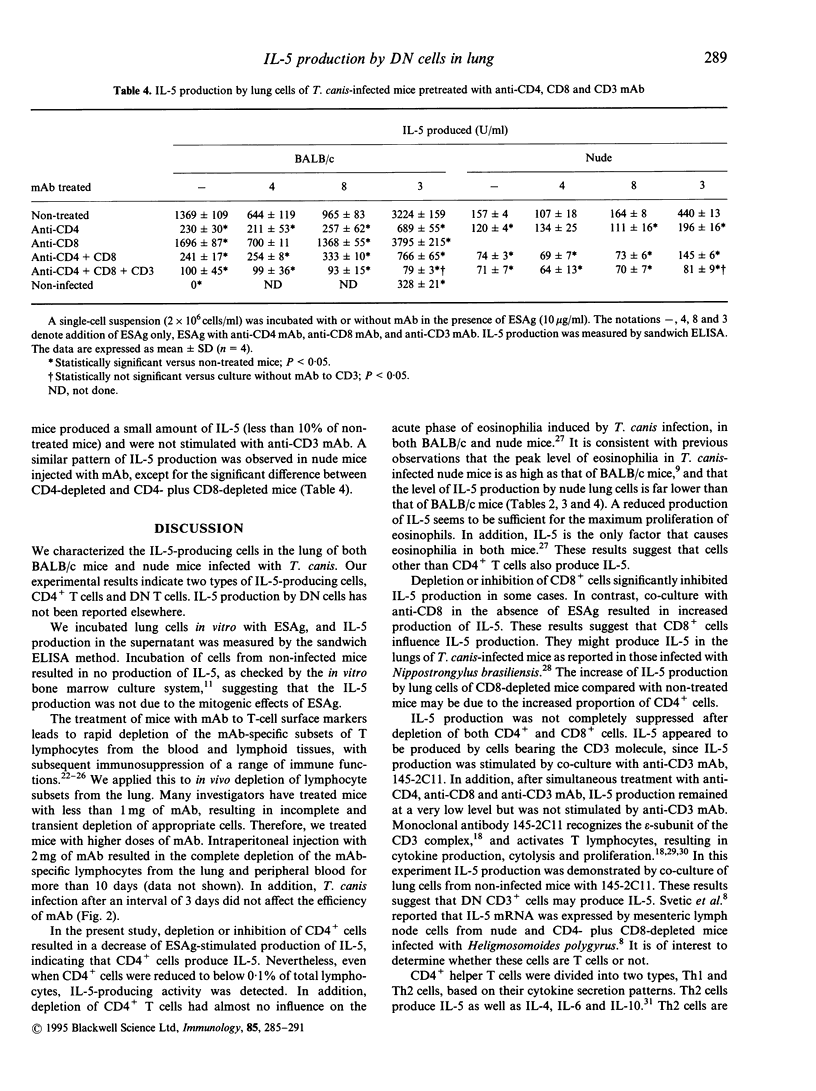
We studied cells in the lungs of BALB/c and BALB/c-nu/nu (nude) mice infected with Toxocara canis, which produced interleukin-5 (IL-5) in in vitro culture with larval excretory-secretory antigen (ESAg). The proportion of CD4+/CD8+/CD4- CD8- cells in lungs of both BALB/c and nude mice was unchanged before and after infection with T. canis. Panning and complement-mediated lysis using monoclonal antibody (mAb) to CD4 showed that CD4+ cells in the lung from both mice produced IL-5. Anti-CD4 mAb suppressed ESAg-stimulated IL-5 production in vitro. In vitro depletion or inhibition of CD8+ cells reduced IL-5 production significantly in some cases, suggesting involvement with IL-5 production. Anti-CD3 mAb enhanced IL-5 production when incubated with or without ESAg. Production of IL-5 was reduced by in vivo depletion of CD4+ cells only and both CD4+ and CD8+ T cells, by intraperitoneal injection with appropriate mAb; IL-5 production was stimulated by anti-CD3 mAb. In contrast, IL-5 production by lung cells of BALB/c mice decreased by more than 90% after simultaneous injection with anti-CD4, anti-CD8 and anti-CD3 mAb, and was not enhanced by anti-CD3 mAb. Similar results were obtained in nude mice. These results suggest that CD4- CD8- T cells, as well as CD4+ T cells, produce IL-5.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustin A., Kubo R. T., Sim G. K. Resident pulmonary lymphocytes expressing the gamma/delta T-cell receptor. Nature. 1989 Jul 20;340(6230):239–241. doi: 10.1038/340239a0. [DOI] [PubMed] [Google Scholar]
- Basten A., Beeson P. B. Mechanism of eosinophilia. II. Role of the lymphocyte. J Exp Med. 1970 Jun 1;131(6):1288–1305. doi: 10.1084/jem.131.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Corrigan C. J., Kay A. B. T cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992 Dec;13(12):501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Field E. H., Rouse T. M., Fleming A. L., Jamali I., Cowdery J. S. Altered IFN-gamma and IL-4 pattern lymphokine secretion in mice partially depleted of CD4 T cells by anti-CD4 monoclonal antibody. J Immunol. 1992 Aug 15;149(4):1131–1137. [PubMed] [Google Scholar]
- Finkelman F. D., Pearce E. J., Urban J. F., Jr, Sher A. Regulation and biological function of helminth-induced cytokine responses. Immunol Today. 1991 Mar;12(3):A62–A66. doi: 10.1016/S0167-5699(05)80018-0. [DOI] [PubMed] [Google Scholar]
- Grencis R. K., Hültner L., Else K. J. Host protective immunity to Trichinella spiralis in mice: activation of Th cell subsets and lymphokine secretion in mice expressing different response phenotypes. Immunology. 1991 Oct;74(2):329–332. [PMC free article] [PubMed] [Google Scholar]
- Grzych J. M., Pearce E., Cheever A., Caulada Z. A., Caspar P., Heiny S., Lewis F., Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991 Feb 15;146(4):1322–1327. [PubMed] [Google Scholar]
- Harada N., Takahashi T., Matsumoto M., Kinashi T., Ohara J., Kikuchi Y., Koyama N., Severinson E., Yaoita Y., Honjo T. Production of a monoclonal antibody useful in the molecular characterization of murine T-cell-replacing factor/B-cell growth factor II. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4581–4585. doi: 10.1073/pnas.84.13.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Jones B., Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988 Mar;9(3):73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- Jarrett E., Ferguson A. Effect of T cell depletion on the potentiated reagin response. Nature. 1974 Aug 2;250(465):420–422. doi: 10.1038/250420a0. [DOI] [PubMed] [Google Scholar]
- Katona I. M., Urban J. F., Jr, Finkelman F. D. The role of L3T4+ and Lyt-2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis. J Immunol. 1988 May 1;140(9):3206–3211. [PubMed] [Google Scholar]
- Koning F., Stingl G., Yokoyama W. M., Yamada H., Maloy W. L., Tschachler E., Shevach E. M., Coligan J. E. Identification of a T3-associated gamma delta T cell receptor on Thy-1+ dendritic epidermal Cell lines. Science. 1987 May 15;236(4803):834–837. doi: 10.1126/science.2883729. [DOI] [PubMed] [Google Scholar]
- Kopf M., Le Gros G., Bachmann M., Lamers M. C., Bluethmann H., Köhler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993 Mar 18;362(6417):245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- Kubota H., Okazaki H., Onuma M., Kano S., Hattori M., Minato N. CD3+4-8- alpha beta T cell population with biased T cell receptor V gene usage. Presence in bone marrow and possible involvement of IL-3 for their extrathymic development. J Immunol. 1992 Aug 15;149(4):1143–1150. [PubMed] [Google Scholar]
- Kusama Y., Takamoto M., Kasahara T., Takatsu K., Nariuchi H., Sugane K. Mechanisms of eosinophilia in BALB/c-nu/+ and congenitally athymic BALB/c-nu/nu mice infected with Toxocara canis. Immunology. 1995 Mar;84(3):461–468. [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley R. L., Styre D., Klein J. R. Differentiation and functional maturation of bone marrow-derived intestinal epithelial T cells expressing membrane T cell receptor in athymic radiation chimeras. J Immunol. 1990 Sep 1;145(5):1369–1375. [PubMed] [Google Scholar]
- Patarca R., Wei F. Y., Singh P., Morasso M. I., Cantor H. Dysregulated expression of the T cell cytokine Eta-1 in CD4-8- lymphocytes during the development of murine autoimmune disease. J Exp Med. 1990 Oct 1;172(4):1177–1183. doi: 10.1084/jem.172.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. M., DiConza J. J., Gold J. A., Reid W. A. Schistosomiasis in the congenitally athymic (nude) mouse. I. Thymic dependency of eosinophilia, granuloma formation, and host morbidity. J Immunol. 1977 Feb;118(2):594–599. [PubMed] [Google Scholar]
- Phillips S. M., Lin J. J., Galal N., Tung A. S., Linette G. P., Perrin P. J. Resistance in murine schistosomiasis is contingent on activated IL-2 receptor-bearing L3T4+ lymphocytes, negatively regulated by Lyt-2+ cells, and uninfluenced by the presence of IL-4. J Immunol. 1991 Feb 15;146(4):1335–1340. [PubMed] [Google Scholar]
- Portoles P., Rojo J., Golby A., Bonneville M., Gromkowski S., Greenbaum L., Janeway C. A., Jr, Murphy D. B., Bottomly K. Monoclonal antibodies to murine CD3 epsilon define distinct epitopes, one of which may interact with CD4 during T cell activation. J Immunol. 1989 Jun 15;142(12):4169–4175. [PubMed] [Google Scholar]
- Ruitenberg E. J., Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976 Nov 18;264(5583):258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- Savigny D. H. In vitro maintenance of Toxocara canis larvae and a simple method for the production of Toxocara ES antigen for use in serodiagnostic tests for visceral larva migrans. J Parasitol. 1975 Aug;61(4):781–782. [PubMed] [Google Scholar]
- Sugane K., Oshima T. Eosinophilia, granuloma formation and migratory behaviour of larvae in the congenitally athymic mouse infected with Toxocara canis. Parasite Immunol. 1982 Sep;4(5):307–318. doi: 10.1111/j.1365-3024.1982.tb00442.x. [DOI] [PubMed] [Google Scholar]
- Svetić A., Madden K. B., Zhou X. D., Lu P., Katona I. M., Finkelman F. D., Urban J. F., Jr, Gause W. C. A primary intestinal helminthic infection rapidly induces a gut-associated elevation of Th2-associated cytokines and IL-3. J Immunol. 1993 Apr 15;150(8 Pt 1):3434–3441. [PubMed] [Google Scholar]
- Takamoto M., Sugane K. Mechanisms of eosinophilia in Toxocara canis infected mice: in vitro production of interleukin 5 by lung cells of both normal and congenitally athymic nude mice. Parasite Immunol. 1993 Sep;15(9):493–500. doi: 10.1111/j.1365-3024.1993.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Tsuchida M., Iiai T., Watanabe H., Abo T. Relative resistance of intermediate TCR cells to anti-CD3 mAb in mice in vivo and their partial functional characterization. Cell Immunol. 1992 Nov;145(1):78–90. doi: 10.1016/0008-8749(92)90314-f. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Mayes D. C., Woodcock J., Seaman W. E. Inhibition of humoral immunity in vivo by monoclonal antibody to L3T4: studies with soluble antigens in intact mice. J Immunol. 1985 Sep;135(3):1698–1701. [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Matsui T., Kasahara T., Etoh S., Tominaga A., Takatsu K., Miura Y., Suda T. In vivo changes of hemopoietic progenitors and the expression of the interleukin 5 gene in eosinophilic mice infected with Toxocara canis. Exp Hematol. 1990 Dec;18(11):1152–1157. [PubMed] [Google Scholar]


