Abstract
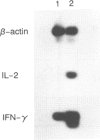
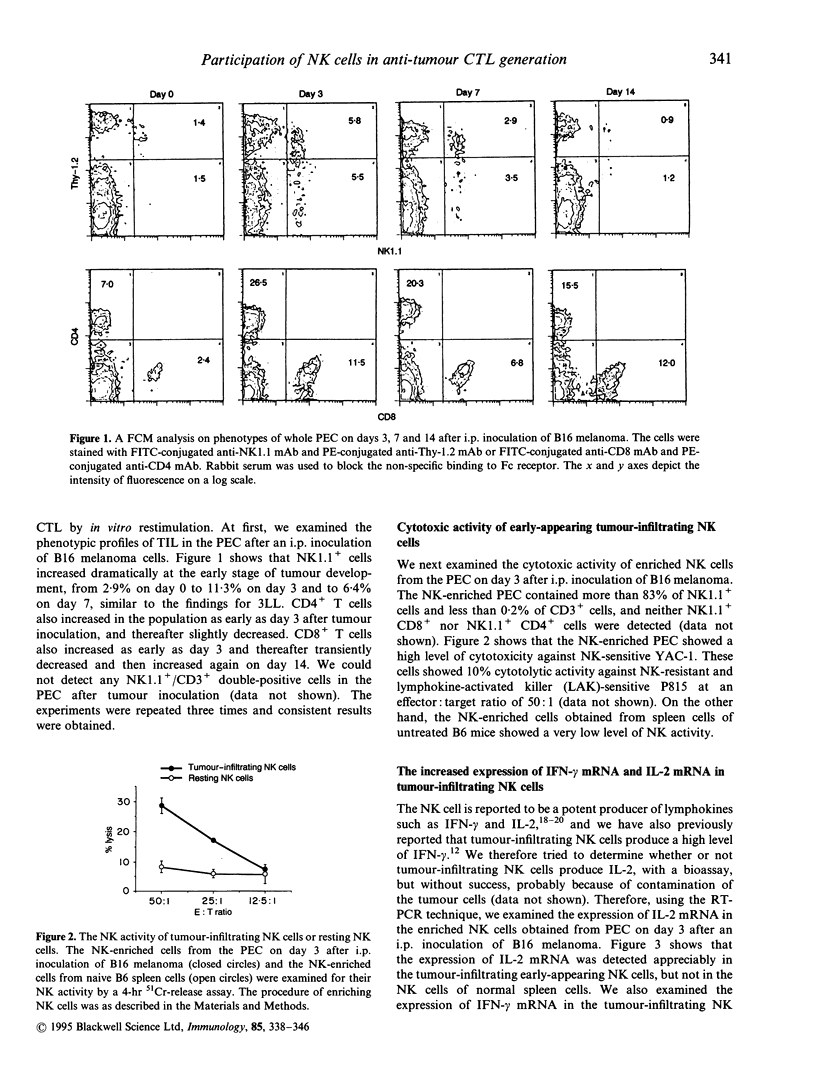
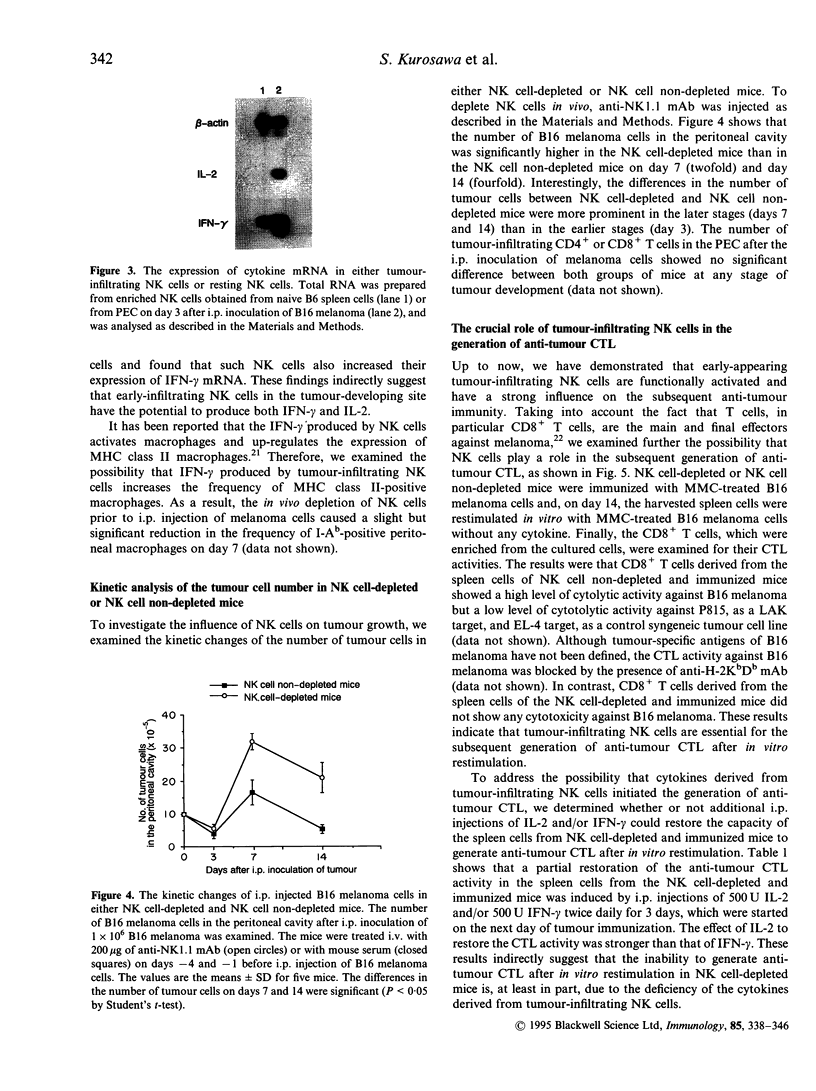
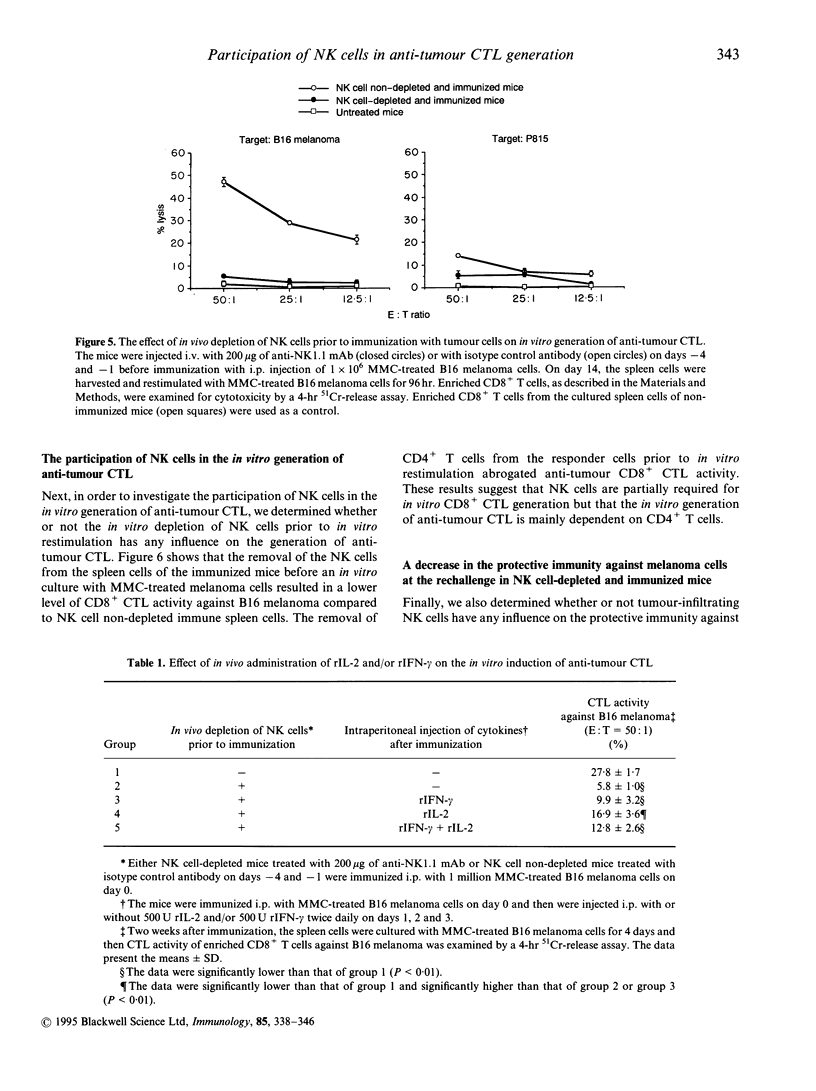
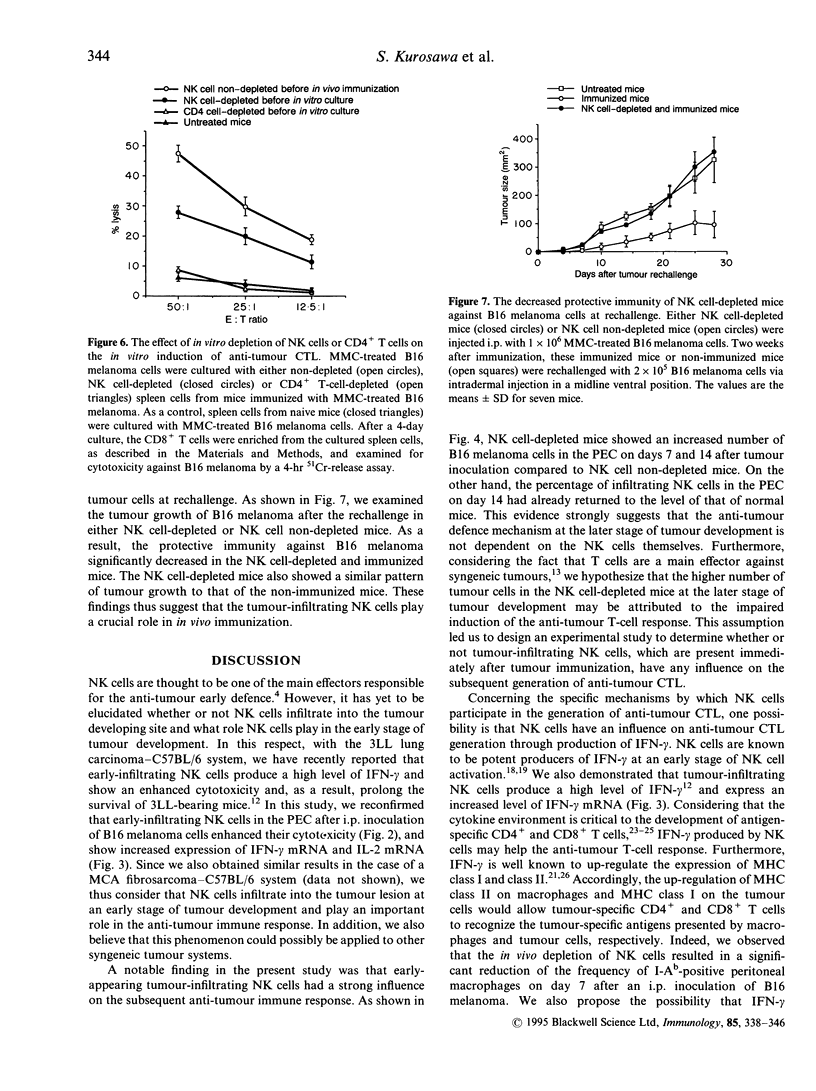
Natural killer (NK) cells that infiltrated into the primary tumour site at an early stage of tumour development, were examined for their participation in the generation of anti-tumour cytotoxic T lymphocytes (CTL). NK cells, which were detected by anti-NK1.1 monoclonal antibody (mAb), increased in the peritoneal exudate cells (PEC) on days 3 and 7 after an intraperitoneal (i.p.) inoculation of syngeneic B16 melanoma cells. These tumour-infiltrating NK cells showed a high level of cytotoxic activity against NK-sensitive YAC-1 cells and an increased expression of interferon-gamma (IFN-gamma) mRNA and interleukin-2 (IL-2) mRNA. The in vivo depletion of NK cells with anti-NK1.1 mAb, prior to i.p. inoculation of B16 melanoma cells, resulted in an increased number of tumour cells in the PEC compared to NK cell non-depleted mice. Interestingly, the differences in tumour cell number between both groups were more prominent on days 7 and 14 than on day 3, which strongly suggested that early-infiltrating NK cells have a large influence on the subsequent anti-tumour response. The in vivo depletion of NK cells prior to immunization with melanoma cells abrogated the capacity of the spleen cells to generate CD8+ tumour-specific CTL after in vitro restimulation. This inability of generating anti-tumour CTL was partially restored by additional i.p. injections of recombinant IL-2 and/or IFN-gamma simultaneously with the immunization of melanoma cells. The in vitro depletion of NK cells prior to the in vitro restimulation with melanoma cells partially impaired the anti-tumour CTL generation from the spleen cells of the immunized mice. Lastly, the in vivo depletion of NK cells prior to immunization with melanoma cells abolished the protective immunity against melanoma cells at the rechallenge. Overall, these results indicate that early-appearing tumour-infiltrating NK cells not only participate in the anti-tumour early defence by themselves, but also play a crucial role in the generation of anti-tumour CTL.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Sheehan K. C., Schreiber R. D., Unanue E. R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989 Jul 1;143(1):127–130. [PubMed] [Google Scholar]
- Blieden T. M., McAdam A. J., Frelinger J. G., Lord E. M. Mechanism of cytolytic T lymphocyte killing of a low class I-expressing tumor. J Immunol. 1991 Aug 15;147(4):1433–1438. [PubMed] [Google Scholar]
- Bonavida B., Katz J., Hoshino T. Mechanism of NK activation by OK-432 (Streptococcus pyogenes). I. Spontaneous release of NKCF and augmentation of NKCF production following stimulation with NK target cells. Cell Immunol. 1986 Oct 1;102(1):126–135. doi: 10.1016/0008-8749(86)90331-x. [DOI] [PubMed] [Google Scholar]
- Chen L., Linsley P. S., Hellström K. E. Costimulation of T cells for tumor immunity. Immunol Today. 1993 Oct;14(10):483–486. doi: 10.1016/0167-5699(93)90262-J. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Pardoll D. M., Itaya T., Golumbek P., Levitsky H. I., Simons J. W., Karasuyama H., Vogelstein B., Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990 Feb 9;60(3):397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- Gansbacher B., Zier K., Daniels B., Cronin K., Bannerji R., Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990 Oct 1;172(4):1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli R. T., Hieny S., Wynn T. A., Wolf S., Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg P. D. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- Habu S., Fukui H., Shimamura K., Kasai M., Nagai Y., Okumura K., Tamaoki N. In vivo effects of anti-asialo GM1. I. Reduction of NK activity and enhancement of transplanted tumor growth in nude mice. J Immunol. 1981 Jul;127(1):34–38. [PubMed] [Google Scholar]
- Harada M., Matsuzaki G., Yoshikai Y., Kobayashi N., Kurosawa S., Takimoto H., Nomoto K. Autoreactive and heat shock protein 60-recognizing CD4+ T-cells show antitumor activity against syngeneic fibrosarcoma. Cancer Res. 1993 Jan 1;53(1):106–111. [PubMed] [Google Scholar]
- Itoh K., Platsoucas C. D., Balch C. M. Autologous tumor-specific cytotoxic T lymphocytes in the infiltrate of human metastatic melanomas. Activation by interleukin 2 and autologous tumor cells, and involvement of the T cell receptor. J Exp Med. 1988 Oct 1;168(4):1419–1441. doi: 10.1084/jem.168.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara T., Djeu J. Y., Dougherty S. F., Oppenheim J. J. Capacity of human large granular lymphocytes (LGL) to produce multiple lymphokines: interleukin 2, interferon, and colony stimulating factor. J Immunol. 1983 Nov;131(5):2379–2385. [PubMed] [Google Scholar]
- Kawano Y., Taniguchi K., Toshitani A., Nomoto K. Synergistic defense system by cooperative natural effectors against metastasis of B16 melanoma cells in H-2-associated control: different behavior of H-2+ and H-2- cells in metastatic processes. J Immunol. 1986 Jun 15;136(12):4729–4734. [PubMed] [Google Scholar]
- Kern D. E., Klarnet J. P., Jensen M. C., Greenberg P. D. Requirement for recognition of class II molecules and processed tumor antigen for optimal generation of syngeneic tumor-specific class I-restricted CTL. J Immunol. 1986 Jun 1;136(11):4303–4310. [PubMed] [Google Scholar]
- Kurosawa S., Matsuzaki G., Harada M., Ando T., Nomoto K. Early appearance and activation of natural killer cells in tumor-infiltrating lymphoid cells during tumor development. Eur J Immunol. 1993 May;23(5):1029–1033. doi: 10.1002/eji.1830230507. [DOI] [PubMed] [Google Scholar]
- Kärre K., Klein G. O., Kiessling R., Klein G., Roder J. C. In vitro NK-activity and in vivo resistance to leukemia: studies of beige, beige//nude and wild-type hosts on C57BL background. Int J Cancer. 1980 Dec 15;26(6):789–797. doi: 10.1002/ijc.2910260613. [DOI] [PubMed] [Google Scholar]
- Nabel G., Allard W. J., Cantor H. A cloned cell line mediating natural killer cell function inhibits immunoglobulin secretion. J Exp Med. 1982 Aug 1;156(2):658–663. doi: 10.1084/jem.156.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkatti M., Clary S. R., Nagarkatti P. S. Characterization of tumor-infiltrating CD4+ T cells as Th1 cells based on lymphokine secretion and functional properties. J Immunol. 1990 Jun 15;144(12):4898–4905. [PubMed] [Google Scholar]
- Ortaldo J. R., Herberman R. B. Heterogeneity of natural killer cells. Annu Rev Immunol. 1984;2:359–394. doi: 10.1146/annurev.iy.02.040184.002043. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T. Cancer immunotherapy using interleukin-2 and interleukin-2-activated lymphocytes. Annu Rev Immunol. 1986;4:681–709. doi: 10.1146/annurev.iy.04.040186.003341. [DOI] [PubMed] [Google Scholar]
- Seaman W. E., Sleisenger M., Eriksson E., Koo G. C. Depletion of natural killer cells in mice by monoclonal antibody to NK-1.1. Reduction in host defense against malignancy without loss of cellular or humoral immunity. J Immunol. 1987 Jun 15;138(12):4539–4544. [PubMed] [Google Scholar]
- Talmadge J. E., Meyers K. M., Prieur D. J., Starkey J. R. Role of NK cells in tumour growth and metastasis in beige mice. Nature. 1980 Apr 17;284(5757):622–624. doi: 10.1038/284622a0. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. F., Dennert G. Effects of a cloned cell line with NK activity on bone marrow transplants, tumour development and metastasis in vivo. Nature. 1982 Nov 4;300(5887):31–34. doi: 10.1038/300031a0. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Kuribayashi K., Miyatake S., Nishihara K., Nakayama E., Taniyama T., Sakata T. Exogenous expression of mouse interferon gamma cDNA in mouse neuroblastoma C1300 cells results in reduced tumorigenicity by augmented anti-tumor immunity. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9456–9460. doi: 10.1073/pnas.86.23.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. A., Ortaldo J. R. One-signal requirement for interferon-gamma production by human large granular lymphocytes. J Immunol. 1987 Aug 1;139(3):724–727. [PubMed] [Google Scholar]