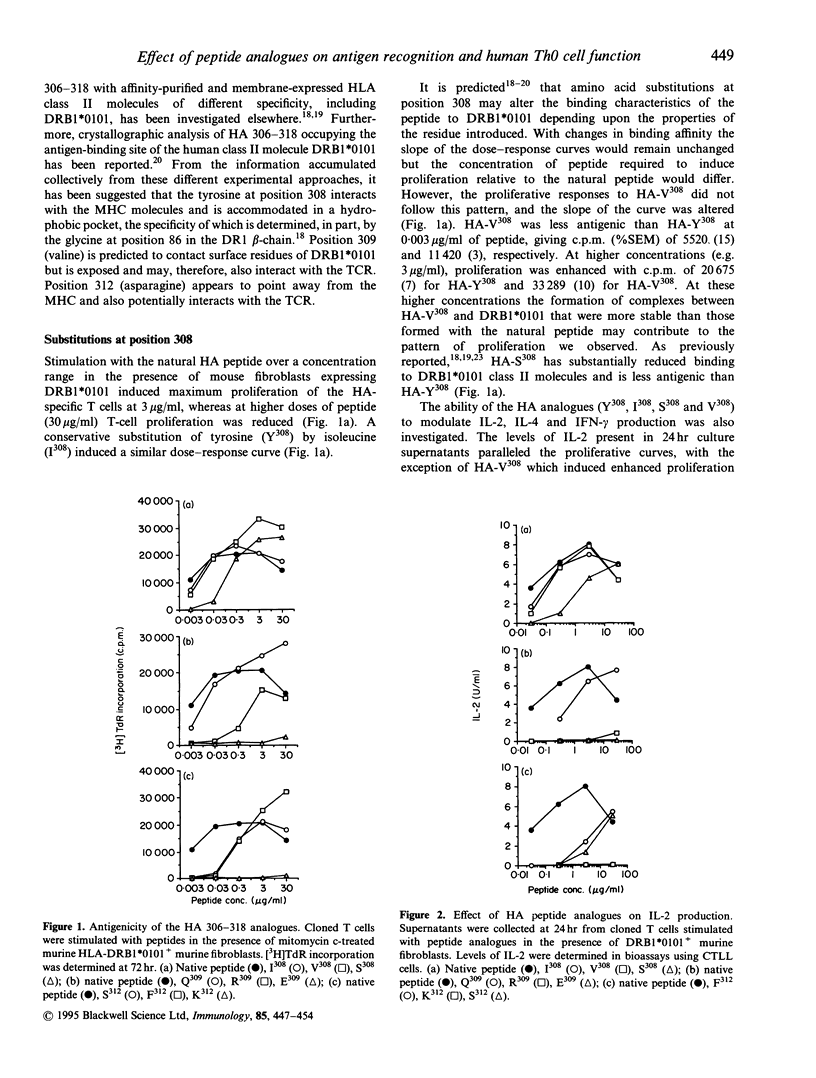
Abstract
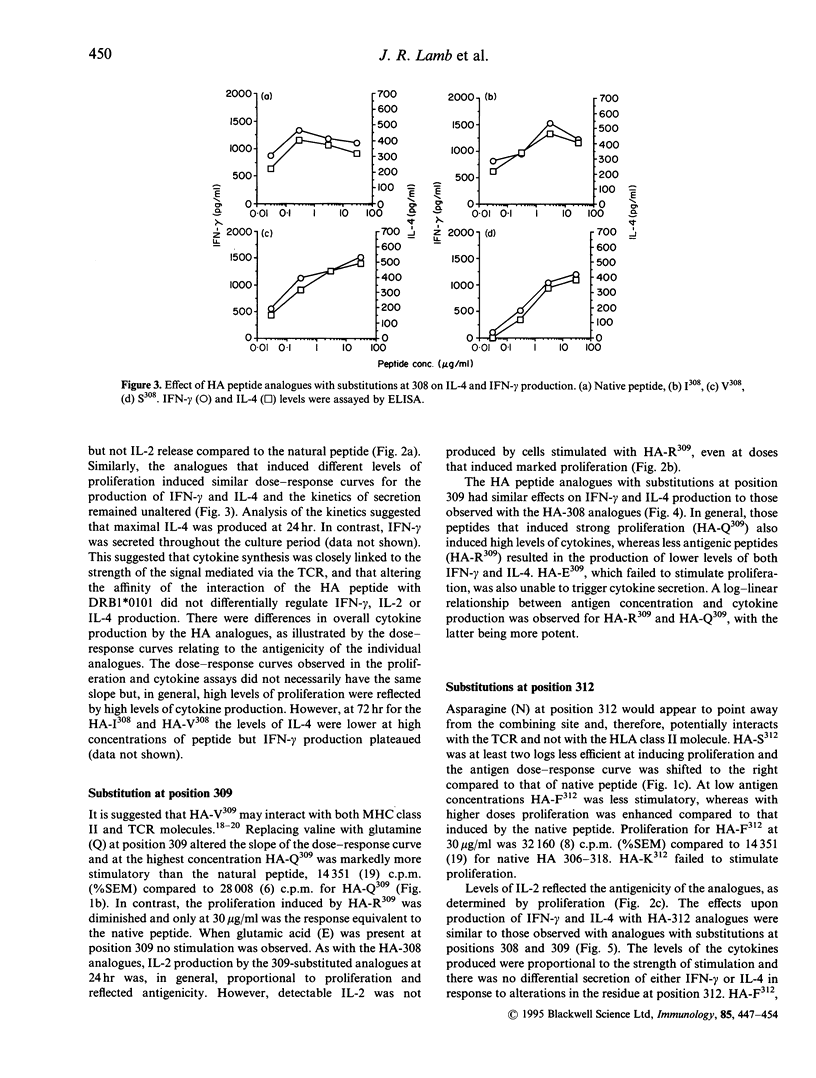
Cytokines can influence the selection of functional subsets (Th1 or Th2) of CD4+ T cells. However, quantitative changes in affinity of peptide/major histocompatibility complex (MHC) class II/T-cell receptor (TCR) interactions may alter antigen density and modulate T-cell effector function. The possibility exists to use peptide analogues to induce a partial signal to dissociate production of interleukin-4 (IL-4) and interferon-gamma (IFN-gamma) by T-helper type-0 (Th0) cells and, consequently, to regulate T-cell function. Based on binding assays and resolution of the crystalline structure of an influenza virus haemagglutinin peptide (HA 306-318) bound to the human MHC class II molecule DRB1*0101, we synthesized HA peptide analogues with amino acid substitutions predicted to modify either MHC class II/peptide density or TCR/peptide interactions. When we examined their antigenicity using cloned human Th0 cells, the analogues, in general, elicited a gradation in potency reflected by a reduction in both proliferation and cytokine production (IL-2, IL-4 and IFN-gamma). Although the analogue HA-R309 diminished IL-2 production, none of the analogues tested could selectively induce only IL-4 or IFN-gamma. Since, in general, the effector functions of the Th0 cells examined here were resistant to selective manipulation by the peptide analogues, this suggests that for some clones of chronically activated T cells modulation of selected functions may be difficult to achieve.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antigenic requirements for activation of MHC restricted responses. Immunol Rev. 1987 Aug;98:1–187. [PubMed] [Google Scholar]
- Busch R., Hill C. M., Hayball J. D., Lamb J. R., Rothbard J. B. Effect of natural polymorphism at residue 86 of the HLA-DR beta chain on peptide binding. J Immunol. 1991 Aug 15;147(4):1292–1298. [PubMed] [Google Scholar]
- Busch R., Rothbard J. B. Detection of peptide-MHC class II complexes on the surface of intact cells. J Immunol Methods. 1990 Nov 6;134(1):1–22. doi: 10.1016/0022-1759(90)90107-7. [DOI] [PubMed] [Google Scholar]
- Chen W., McCluskey J., Rodda S., Carbone F. R. Changes at peptide residues buried in the major histocompatibility complex (MHC) class I binding cleft influence T cell recognition: a possible role for indirect conformational alterations in the MHC class I or bound peptide in determining T cell recognition. J Exp Med. 1993 Mar 1;177(3):869–873. doi: 10.1084/jem.177.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Evavold B. D., Allen P. M. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science. 1991 May 31;252(5010):1308–1310. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]
- Evavold B. D., Sloan-Lancaster J., Allen P. M. Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunol Today. 1993 Dec;14(12):602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Pinnas M., Wong T., Fitch F. W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991 Mar 15;146(6):1750–1758. [PubMed] [Google Scholar]
- Hsieh C. S., Heimberger A. B., Gold J. S., O'Garra A., Murphy K. M. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardetzky T. S., Gorga J. C., Busch R., Rothbard J., Strominger J. L., Wiley D. C. Peptide binding to HLA-DR1: a peptide with most residues substituted to alanine retains MHC binding. EMBO J. 1990 Jun;9(6):1797–1803. doi: 10.1002/j.1460-2075.1990.tb08304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsenberg M. L., Wierenga E. A., Bos J. D., Jansen H. M. Functional subsets of allergen-reactive human CD4+ T cells. Immunol Today. 1991 Nov;12(11):392–395. doi: 10.1016/0167-5699(91)90137-I. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Eckels D. D., Lake P., Woody J. N., Green N. Human T-cell clones recognize chemically synthesized peptides of influenza haemagglutinin. Nature. 1982 Nov 4;300(5887):66–69. doi: 10.1038/300066a0. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Street N. F., Budd R., O'Garra A., Fong T. A., Bond M. W., Moore K. W., Sher A., Fiorentino D. F. Diversity of cytokine synthesis and function of mouse CD4+ T cells. Immunol Rev. 1991 Oct;123:209–229. doi: 10.1111/j.1600-065x.1991.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Murray J. S., Madri J., Pasqualini T., Bottomly K. Functional CD4 T cell subset interplay in an intact immune system. J Immunol. 1993 May 15;150(10):4270–4276. [PubMed] [Google Scholar]
- Murray J. S., Madri J., Tite J., Carding S. R., Bottomly K. MHC control of CD4+ T cell subset activation. J Exp Med. 1989 Dec 1;170(6):2135–2140. doi: 10.1084/jem.170.6.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hehir R. E., Yssel H., Verma S., de Vries J. E., Spits H., Lamb J. R. Clonal analysis of differential lymphokine production in peptide and superantigen induced T cell anergy. Int Immunol. 1991 Aug;3(8):819–826. doi: 10.1093/intimm/3.8.819. [DOI] [PubMed] [Google Scholar]
- O'Sullivan D., Arrhenius T., Sidney J., Del Guercio M. F., Albertson M., Wall M., Oseroff C., Southwood S., Colón S. M., Gaeta F. C. On the interaction of promiscuous antigenic peptides with different DR alleles. Identification of common structural motifs. J Immunol. 1991 Oct 15;147(8):2663–2669. [PubMed] [Google Scholar]
- Racioppi L., Ronchese F., Matis L. A., Germain R. N. Peptide-major histocompatibility complex class II complexes with mixed agonist/antagonist properties provide evidence for ligand-related differences in T cell receptor-dependent intracellular signaling. J Exp Med. 1993 Apr 1;177(4):1047–1060. doi: 10.1084/jem.177.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard J. B., Lechler R. I., Howland K., Bal V., Eckels D. D., Sekaly R., Long E. O., Taylor W. R., Lamb J. R. Structural model of HLA-DR1 restricted T cell antigen recognition. Cell. 1988 Feb 26;52(4):515–523. doi: 10.1016/0092-8674(88)90464-3. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E., Davis M. M., Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992 Oct 1;176(4):1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloway P., Fish S., Passmore H., Gefter M., Coffee R., Manser T. Regulation of the immune response to peptide antigens: differential induction of immediate-type hypersensitivity and T cell proliferation due to changes in either peptide structure or major histocompatibility complex haplotype. J Exp Med. 1991 Oct 1;174(4):847–858. doi: 10.1084/jem.174.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern L. J., Brown J. H., Jardetzky T. S., Gorga J. C., Urban R. G., Strominger J. L., Wiley D. C. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994 Mar 17;368(6468):215–221. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunol Today. 1993 Jul;14(7):335–338. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- Weaver C. T., Hawrylowicz C. M., Unanue E. R. T helper cell subsets require the expression of distinct costimulatory signals by antigen-presenting cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8181–8185. doi: 10.1073/pnas.85.21.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]


