Abstract
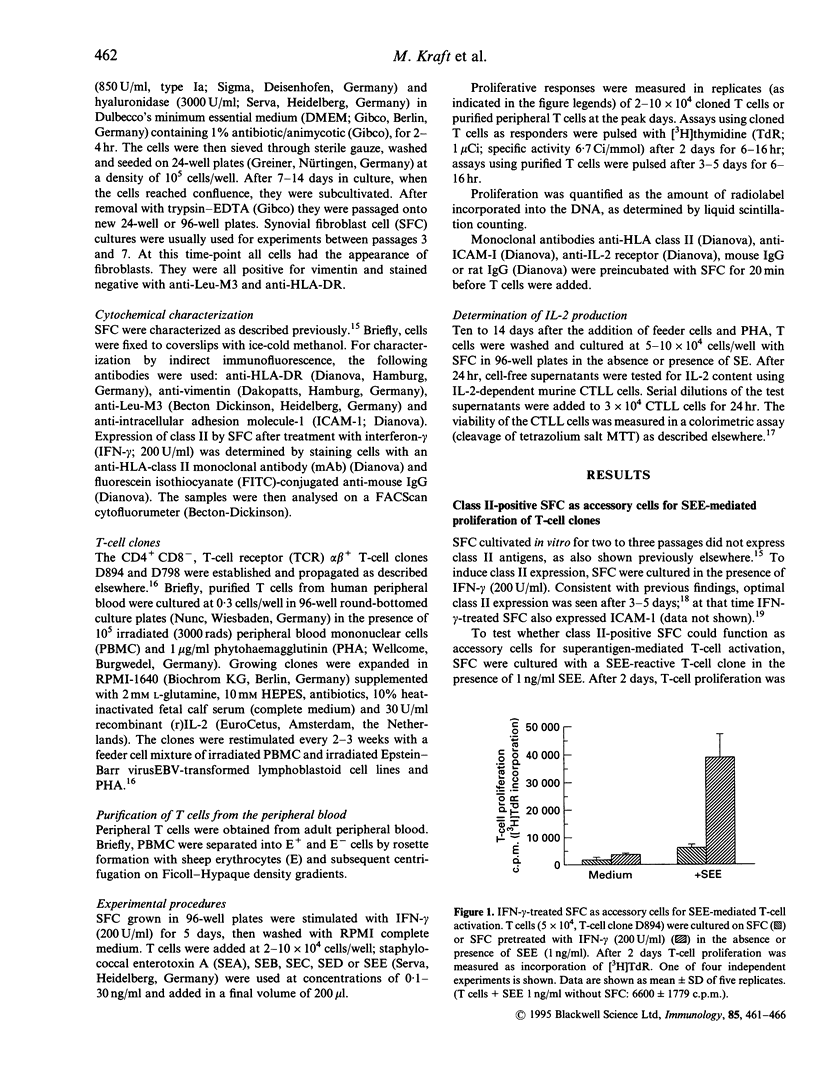
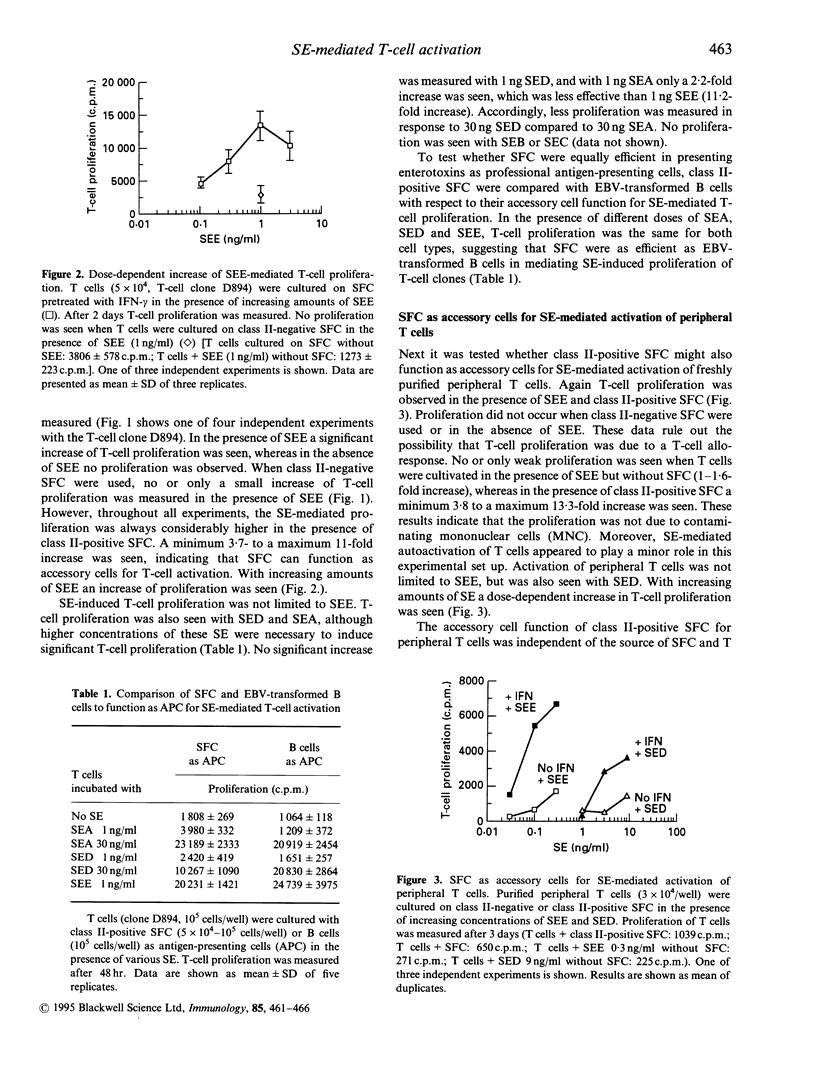
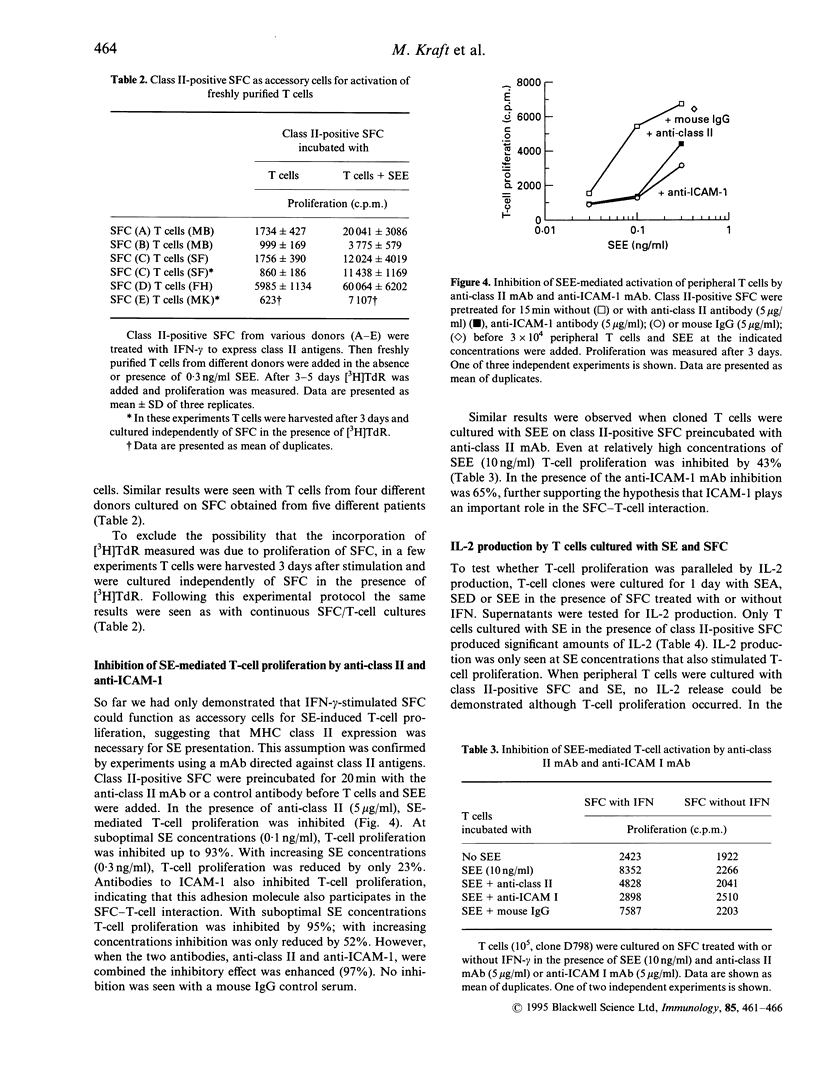
Rheumatoid arthritis (RA) is thought to be the result of T-cell-mediated autoimmune phenomena. So far, a critical autoantigen has not been identified. Recently, superantigens have been implied in the pathogenesis of RA. In the present study it was tested whether major histocompatibility complex (MHC) class II-positive synovial fibroblast cells (SFC) function as superantigen-presenting cells. SFC were stimulated with interferon-gamma (IFN-gamma) to express class II antigens; then they were cultivated in the presence of T cells with or without staphylococcal enterotoxins (SE). T-cell activation was measured as proliferation and interleukin-2 (IL-2) production. Depending on the dose and type of SE, activation of T-cell clones and also of peripheral T cells was seen. T-cell activation was inhibited by antibodies to MHC class II antigens and also by antibodies to intracellular adhesion molecule type-1 (ICAM-1). The data suggest that class II-positive SFC have the capacity to serve as accessory cells for superantigen-mediated T-cell activation. Thus SFC may participate in the propagation of a T-cell dependent immune response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burmester G. R., Jahn B., Gramatzki M., Zacher J., Kalden J. R. Activated T cells in vivo and in vitro: divergence in expression of Tac and Ia antigens in the nonblastoid small T cells of inflammation and normal T cells activated in vitro. J Immunol. 1984 Sep;133(3):1230–1234. [PubMed] [Google Scholar]
- Burmester G. R., Jahn B., Rohwer P., Zacher J., Winchester R. J., Kalden J. R. Differential expression of Ia antigens by rheumatoid synovial lining cells. J Clin Invest. 1987 Sep;80(3):595–604. doi: 10.1172/JCI113111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. E., Winterrowd G. E., Krzesicki R. F., Sanders M. E. Role of cytokines in inflammatory synovitis. The coordinate regulation of intercellular adhesion molecule 1 and HLA class I and class II antigens in rheumatoid synovial fibroblasts. Arthritis Rheum. 1990 Dec;33(12):1776–1786. doi: 10.1002/art.1780331204. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H., Gjörloff A., Hedlund G., Hedman H., Lundgren E., Kalland T., Sjögren H. O., Dohlsten M. Stimulation of human naive and memory T helper cells with bacterial superantigen. Naive CD4+45RA+ T cells require a costimulatory signal mediated through the LFA-1/ICAM-1 pathway. J Immunol. 1992 Apr 1;148(7):1993–1998. [PubMed] [Google Scholar]
- Geppert T. D., Lipsky P. E. Antigen presentation by interferon-gamma-treated endothelial cells and fibroblasts: differential ability to function as antigen-presenting cells despite comparable Ia expression. J Immunol. 1985 Dec;135(6):3750–3762. [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Hazenberg M. P., Klasen I. S., Kool J., Ruseler-van Embden J. G., Severijnen A. J. Are intestinal bacteria involved in the etiology of rheumatoid arthritis? Review article. APMIS. 1992 Jan;100(1):1–9. doi: 10.1111/j.1699-0463.1992.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Herman A., Kappler J. W., Marrack P., Pullen A. M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- Herrmann T., Romero P., Sartoris S., Paiola F., Accolla R. S., Maryanski J. L., MacDonald H. R. Staphylococcal enterotoxin-dependent lysis of MHC class II negative target cells by cytolytic T lymphocytes. J Immunol. 1991 Apr 15;146(8):2504–2512. [PubMed] [Google Scholar]
- Holoshitz J., Matitiau A., Cohen I. R. Arthritis induced in rats by cloned T lymphocytes responsive to mycobacteria but not to collagen type II. J Clin Invest. 1984 Jan;73(1):211–215. doi: 10.1172/JCI111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell M. D., Diveley J. P., Lundeen K. A., Esty A., Winters S. T., Carlo D. J., Brostoff S. W. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10921–10925. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelitz D., Wesselborg S. Life and death of a superantigen-reactive human CD4+ T cell clone: staphylococcal enterotoxins induce death by apoptosis but simultaneously trigger a proliferative response in the presence of HLA-DR+ antigen-presenting cells. Int Immunol. 1992 Dec;4(12):1381–1388. doi: 10.1093/intimm/4.12.1381. [DOI] [PubMed] [Google Scholar]
- Keystone E. C., Minden M., Klock R., Poplonski L., Zalcberg J., Takadera T., Mak T. W. Structure of T cell antigen receptor beta chain in synovial fluid cells from patients with rheumatoid arthritis. Arthritis Rheum. 1988 Dec;31(12):1555–1557. doi: 10.1002/art.1780311213. [DOI] [PubMed] [Google Scholar]
- Koning F., Rust C. Staphylococcal enterotoxin-mediated human T-T cell interactions. J Immunol. 1992 Jul 1;149(1):317–322. [PubMed] [Google Scholar]
- Lindblad S., Klareskog L., Hedfors E., Forsum U., Sundström C. Phenotypic characterization of synovial tissue cells in situ in different types of synovitis. Arthritis Rheum. 1983 Nov;26(11):1321–1332. doi: 10.1002/art.1780261104. [DOI] [PubMed] [Google Scholar]
- McCulloch J., Lydyard P. M., Rook G. A. Rheumatoid arthritis: how well do the theories fit the evidence? Clin Exp Immunol. 1993 Apr;92(1):1–6. doi: 10.1111/j.1365-2249.1993.tb05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltenburg A. M., van Laar J. M., Daha M. R., de Vries R. R., van den Elsen P. J., Breedveld F. C. Dominant T-cell receptor beta-chain gene rearrangements indicate clonal expansion in the rheumatoid joint. Scand J Immunol. 1990 Jan;31(1):121–126. doi: 10.1111/j.1365-3083.1990.tb02750.x. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Mitra R. S., Green J., Zheng X. G., Shimizu Y., Thompson C., Turka L. A. Accessory cell function of keratinocytes for superantigens. Dependence on lymphocyte function-associated antigen-1/intercellular adhesion molecule-1 interaction. J Immunol. 1993 Mar 15;150(6):2148–2159. [PubMed] [Google Scholar]
- Stamenkovic I., Stegagno M., Wright K. A., Krane S. M., Amento E. P., Colvin R. B., Duquesnoy R. J., Kurnick J. T. Clonal dominance among T-lymphocyte infiltrates in arthritis. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1179–1183. doi: 10.1073/pnas.85.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Acha-Orbea H., Bell J. I., Chao N., Fronek Z., Jacob C. O., McDermott M., Sinha A. A., Timmerman L., Steinman L. A molecular basis for MHC class II--associated autoimmunity. Science. 1988 May 20;240(4855):1003–1009. doi: 10.1126/science.3368786. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Araake M., Yan X. J., Miyanaga Y., Igarashi H. Involvement of HLA class II molecules in acquisition of staphylococcal enterotoxin A-binding activity and accessory cell activity in activation of human T cells by related toxins in vascular endothelial cells. Clin Exp Immunol. 1992 Feb;87(2):322–328. doi: 10.1111/j.1365-2249.1992.tb02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu Y., Wege H., Straus A., Ott M., Bannwarth W., Lanchbury J., Panayi G., Steinmetz M. The T-cell-receptor repertoire in the synovial fluid of a patient with rheumatoid arthritis is polyclonal. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8534–8538. doi: 10.1073/pnas.88.19.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kempis J., Torbohm I., Schönermark M., Jahn B., Seitz M., Hänsch G. M. Effect of the late complement components C5b-9 and of platelet-derived growth factor on the prostaglandin release of human synovial fibroblast-like cells. Int Arch Allergy Appl Immunol. 1989;90(3):248–255. doi: 10.1159/000235032. [DOI] [PubMed] [Google Scholar]


