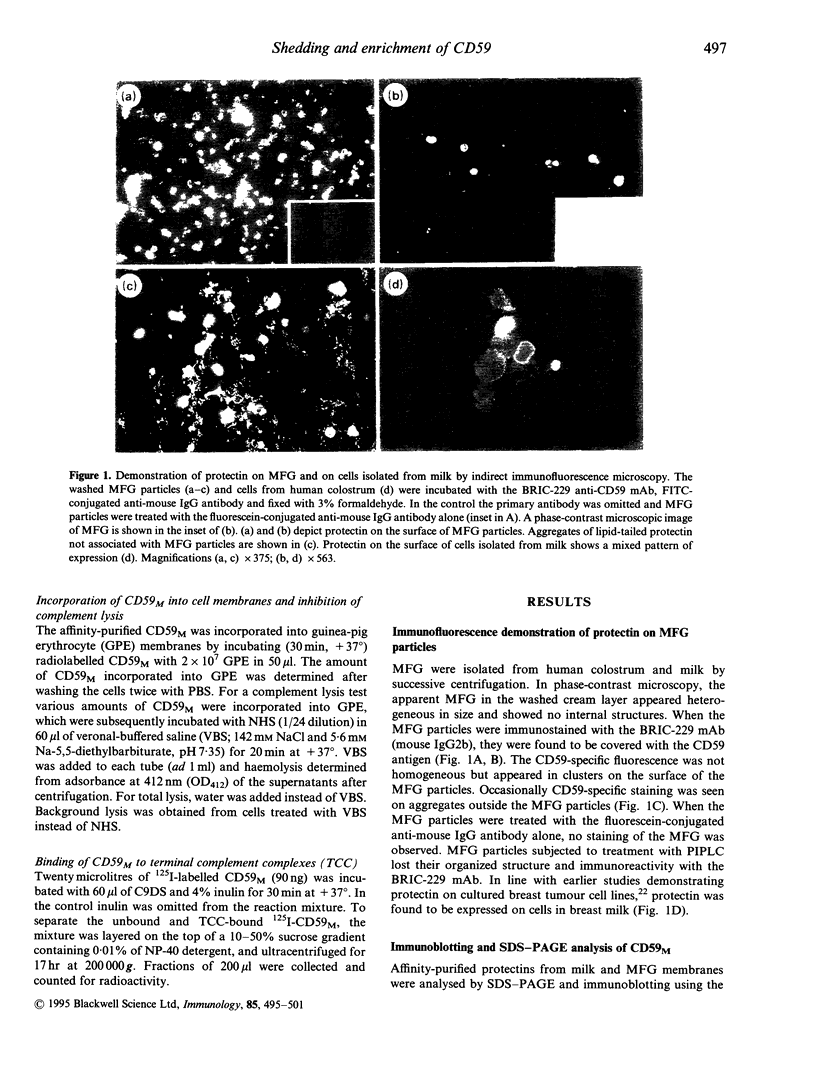
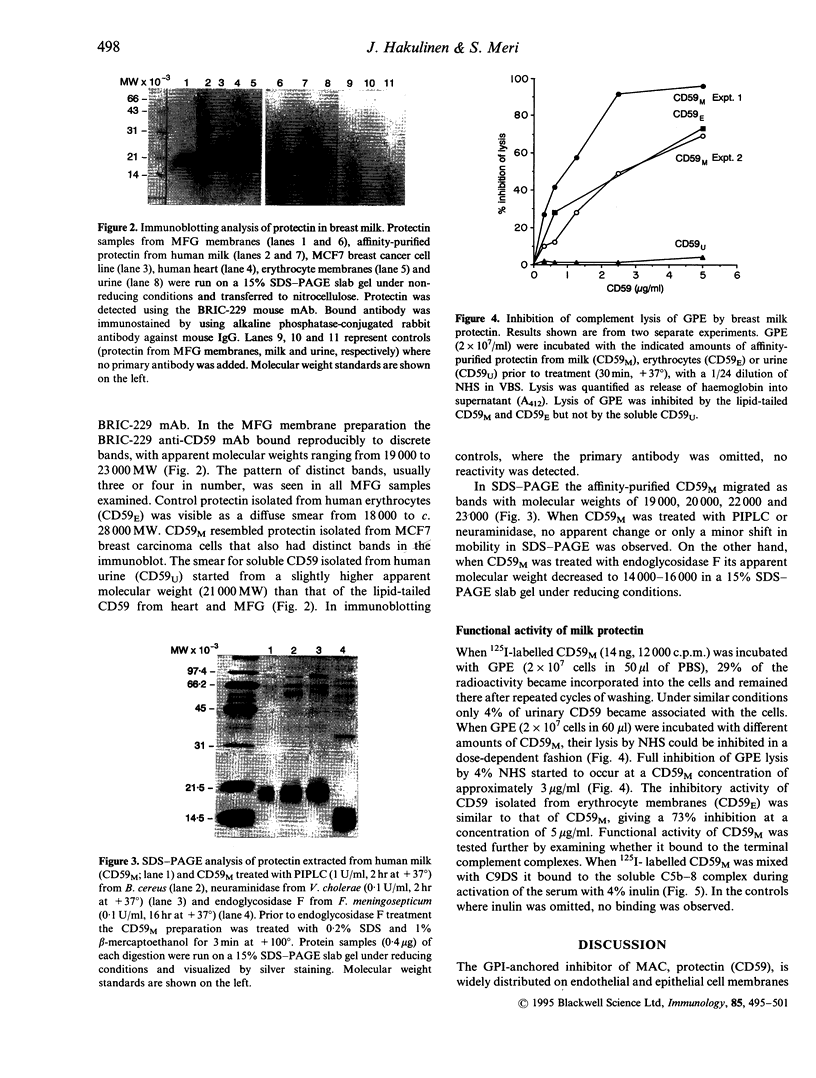
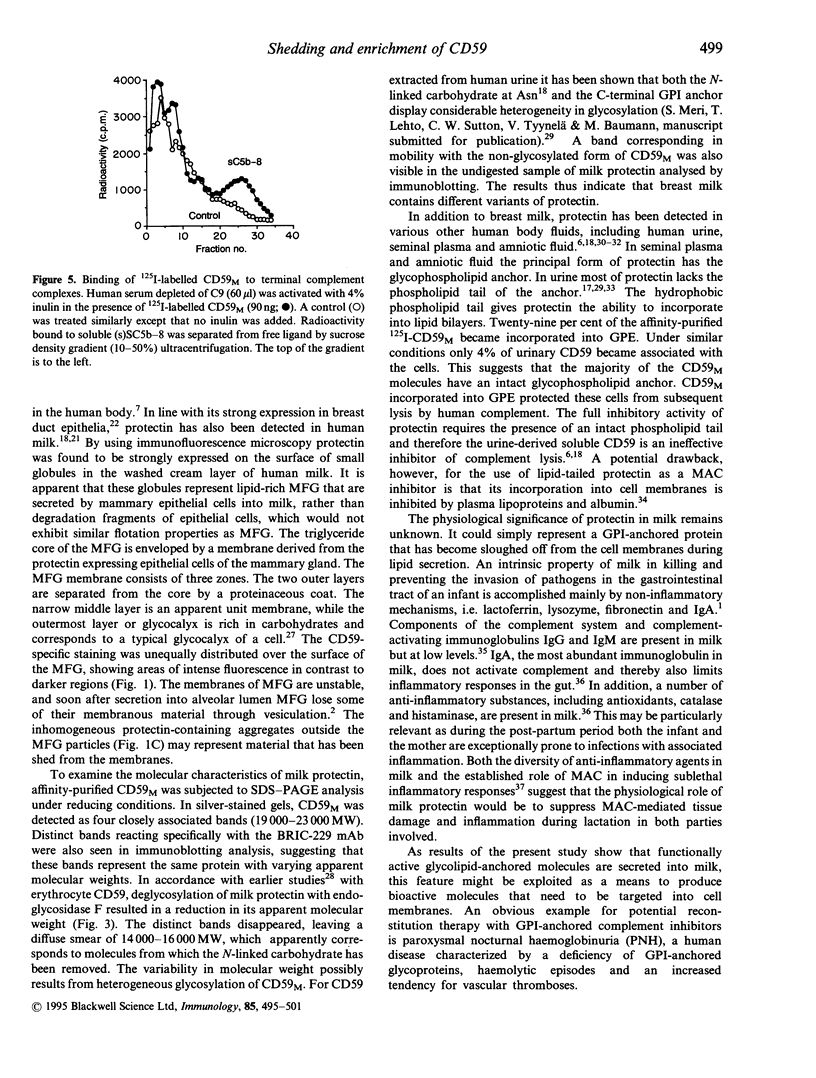
Abstract
Protectin (CD59) is a glycolipid-anchored inhibitor of the membrane attack complex (MAC) of human complement (C) that protects blood cells, endothelial cells and various epithelial cells from C-mediated lysis. Because of its activities protectin is a candidate molecule for use in the treatment of paroxysmal nocturnal haemoglobinuria or conditions where MAC causes tissue damage. Soluble, phospholipid-free forms of protectin have been isolated from human urine and produced in recombinant form, but they have only a relatively weak C lysis-inhibiting activity. In the present study we have looked for functionally active protectin in human breast milk. Milk is rich in fat droplets, milk fat globules (MFG), that are enveloped in a plasma membrane derived from secretory cells of the mammary gland. The membranes of MFG contain a variety of glycoproteins expressed by the mammary epithelial cells. Both immunofluorescence and immunoblotting analysis demonstrated that protectin was strongly expressed on human MFG. In sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis, MFG protectin (CD59M) appeared as distinct bands with apparent molecular weights of 19,000-23,000 MW, similar to protectin extracted from MCF7 breast carcinoma cells. CD59M in breast milk was functionally active and had a glycophospholipid anchor, as judged by its ability to incorporate into guinea-pig erythrocytes and inhibit their lysis by human complement. These results indicate that functionally active protectin becomes enriched in MFG and imply that secretion of glycophospholipid-anchored molecules, e.g. into cow milk and colostrum, could be exploited as a means of producing bioactive molecules that need to be targeted into cell membranes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjørge L., Jensen T. S., Vedeler C. A., Ulvestad E., Kristoffersen E. K., Matre R. Soluble CD59 in pregnancy and infancy. Immunol Lett. 1993 May;36(2):233–233. doi: 10.1016/0165-2478(93)90058-a. [DOI] [PubMed] [Google Scholar]
- Ceriani R. L., Blank E. W. Experimental therapy of human breast tumors with 131I-labeled monoclonal antibodies prepared against the human milk fat globule. Cancer Res. 1988 Aug 15;48(16):4664–4672. [PubMed] [Google Scholar]
- Davies A., Simmons D. L., Hale G., Harrison R. A., Tighe H., Lachmann P. J., Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989 Sep 1;170(3):637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher C. M., Harrison R. A., Lachmann P. J., Neuhaus D. Sequence-specific 1H-NMR assignments and folding topology of human CD59. Protein Sci. 1993 Dec;2(12):2015–2027. doi: 10.1002/pro.5560021203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamm H., Huber C., Chapel H., Lee M., Ries F., Dicato M. A. Intravenous immune globulin in chronic lymphocytic leukaemia. Clin Exp Immunol. 1994 Jul;97 (Suppl 1):17–20. [PMC free article] [PubMed] [Google Scholar]
- Goldman A. S., Thorpe L. W., Goldblum R. M., Hanson L. A. Anti-inflammatory properties of human milk. Acta Paediatr Scand. 1986 Sep;75(5):689–695. doi: 10.1111/j.1651-2227.1986.tb10275.x. [DOI] [PubMed] [Google Scholar]
- Hakulinen J., Meri S. Expression and function of the complement membrane attack complex inhibitor protectin (CD59) on human breast cancer cells. Lab Invest. 1994 Dec;71(6):820–827. [PubMed] [Google Scholar]
- Imam A., Laurence D. J., Neville A. M. Isolation and characterization of a major glycoprotein from milk-fat-globule membrane of human breast milk. Biochem J. 1981 Jan 1;193(1):47–54. doi: 10.1042/bj1930047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehto T., Honkanen E., Teppo A. M., Meri S. Urinary excretion of protectin (CD59), complement SC5b-9 and cytokines in membranous glomerulonephritis. Kidney Int. 1995 May;47(5):1403–1411. doi: 10.1038/ki.1995.197. [DOI] [PubMed] [Google Scholar]
- Lehto T., Meri S. Interactions of soluble CD59 with the terminal complement complexes. CD59 and C9 compete for a nascent epitope on C8. J Immunol. 1993 Nov 1;151(9):4941–4949. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Tolbert N. E., Bieber L. L. Protein determination in membrane and lipoprotein samples: manual and automated procedures. Methods Enzymol. 1981;72:296–303. doi: 10.1016/s0076-6879(81)72018-4. [DOI] [PubMed] [Google Scholar]
- Meri S., Morgan B. P., Davies A., Daniels R. H., Olavesen M. G., Waldmann H., Lachmann P. J. Human protectin (CD59), an 18,000-20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990 Sep;71(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Meri S., Waldmann H., Lachmann P. J. Distribution of protectin (CD59), a complement membrane attack inhibitor, in normal human tissues. Lab Invest. 1991 Nov;65(5):532–537. [PubMed] [Google Scholar]
- Morgan B. P. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J. 1989 Nov 15;264(1):1–14. doi: 10.1042/bj2640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y., Noda K., Endo T., Kobata A., Tomita M. Structural study on the glycosyl-phosphatidylinositol anchor and the asparagine-linked sugar chain of a soluble form of CD59 in human urine. Arch Biochem Biophys. 1994 May 15;311(1):117–126. doi: 10.1006/abbi.1994.1216. [DOI] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Ninomiya H., Sims P. J. The human complement regulatory protein CD59 binds to the alpha-chain of C8 and to the "b"domain of C9. J Biol Chem. 1992 Jul 5;267(19):13675–13680. [PubMed] [Google Scholar]
- Okada N., Harada R., Fujita T., Okada H. A novel membrane glycoprotein capable of inhibiting membrane attack by homologous complement. Int Immunol. 1989;1(2):205–208. doi: 10.1093/intimm/1.2.205. [DOI] [PubMed] [Google Scholar]
- Patton S., Huston G. E. A method for isolation of milk fat globules. Lipids. 1986 Feb;21(2):170–174. doi: 10.1007/BF02534441. [DOI] [PubMed] [Google Scholar]
- Rollins S. A., Sims P. J. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990 May 1;144(9):3478–3483. [PubMed] [Google Scholar]
- Rooney I. A., Atkinson J. P., Krul E. S., Schonfeld G., Polakoski K., Saffitz J. E., Morgan B. P. Physiologic relevance of the membrane attack complex inhibitory protein CD59 in human seminal plasma: CD59 is present on extracellular organelles (prostasomes), binds cell membranes, and inhibits complement-mediated lysis. J Exp Med. 1993 May 1;177(5):1409–1420. doi: 10.1084/jem.177.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney I. A., Morgan B. P. Characterization of the membrane attack complex inhibitory protein CD59 antigen on human amniotic cells and in amniotic fluid. Immunology. 1992 Aug;76(4):541–547. [PMC free article] [PubMed] [Google Scholar]
- Schönermark S., Rauterberg E. W., Shin M. L., Löke S., Roelcke D., Hänsch G. M. Homologous species restriction in lysis of human erythrocytes: a membrane-derived protein with C8-binding capacity functions as an inhibitor. J Immunol. 1986 Mar 1;136(5):1772–1776. [PubMed] [Google Scholar]
- Sugita Y., Nakano Y., Tomita M. Isolation from human erythrocytes of a new membrane protein which inhibits the formation of complement transmembrane channels. J Biochem. 1988 Oct;104(4):633–637. doi: 10.1093/oxfordjournals.jbchem.a122524. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väkevä A., Jauhiainen M., Ehnholm C., Lehto T., Meri S. High-density lipoproteins can act as carriers of glycophosphoinositol lipid-anchored CD59 in human plasma. Immunology. 1994 May;82(1):28–33. [PMC free article] [PubMed] [Google Scholar]
- Welsch U., Schumacher U., Buchheim W., Schinko I., Jenness P., Patton S. Histochemical and biochemical observations on milk-fat-globule membranes from several mammalian species. Acta Histochem Suppl. 1990;40:59–64. [PubMed] [Google Scholar]
- Zalman L. S., Wood L. M., Müller-Eberhard H. J. Isolation of a human erythrocyte membrane protein capable of inhibiting expression of homologous complement transmembrane channels. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6975–6979. doi: 10.1073/pnas.83.18.6975. [DOI] [PMC free article] [PubMed] [Google Scholar]