Abstract
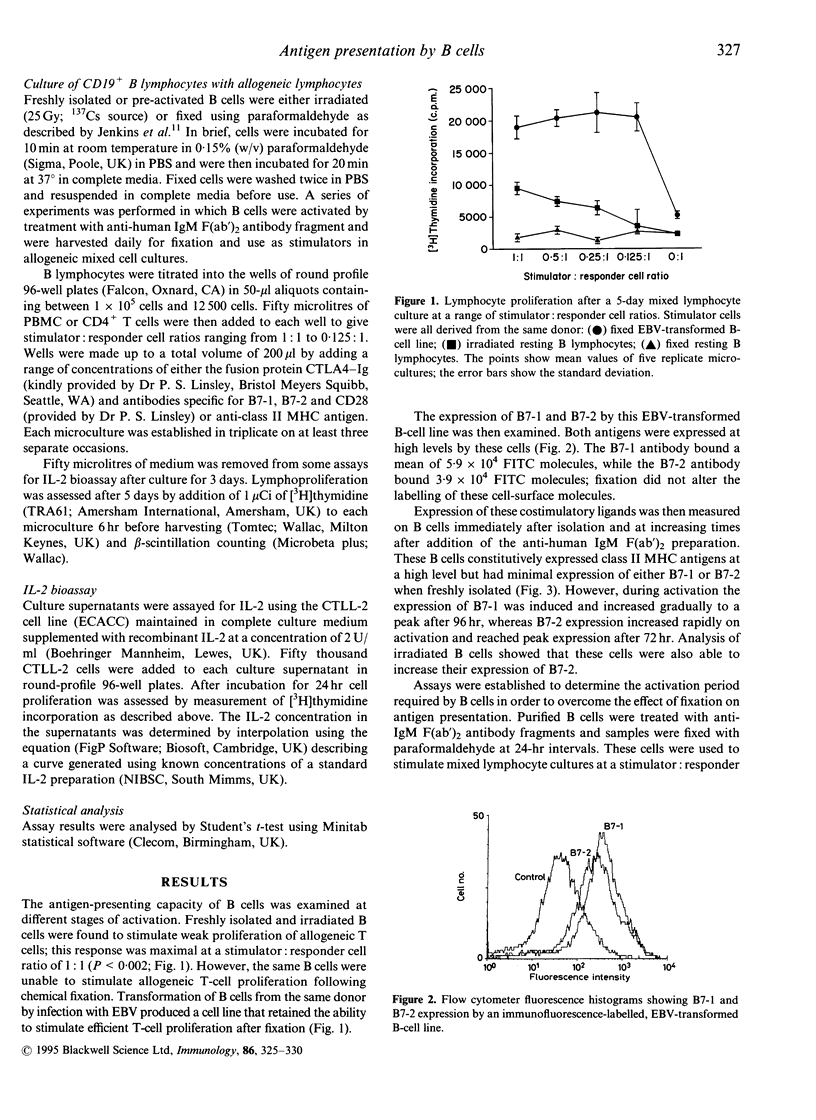
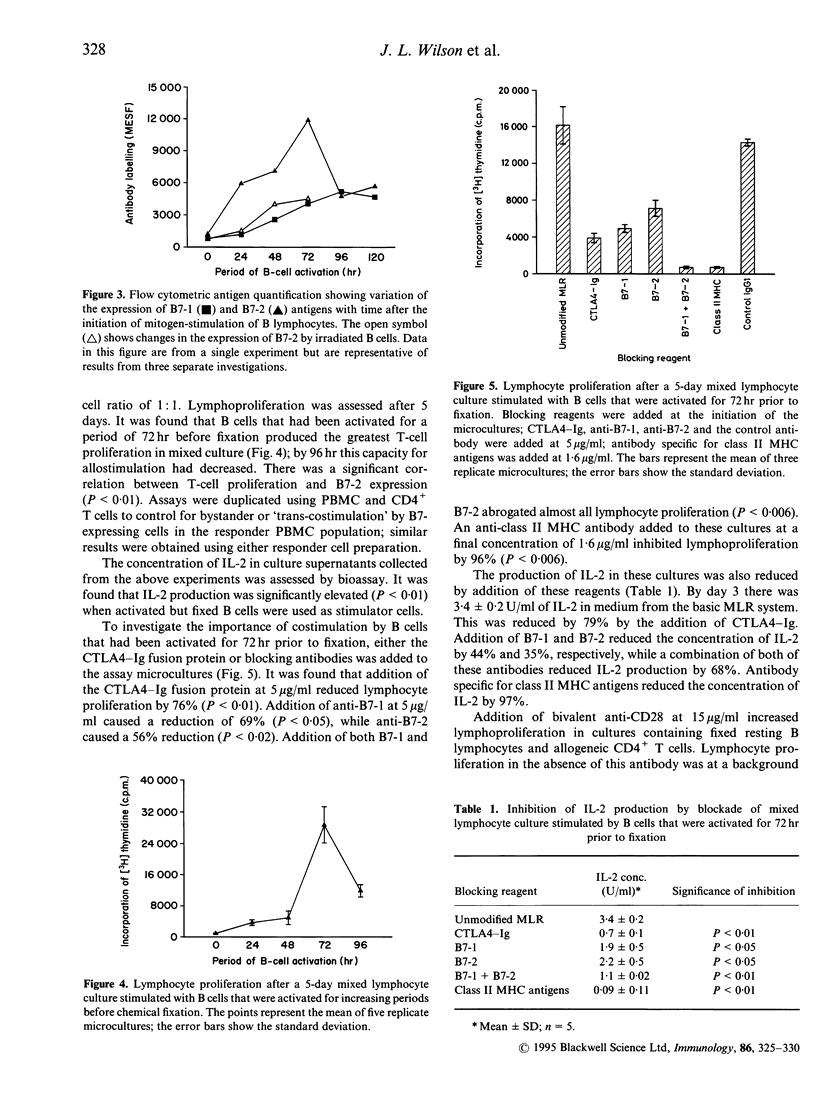
This paper describes a model for investigation of the functional implications of B-cell activation for antigen presentation. Mixed lymphocyte cultures were used to assess the ability of freshly isolated B cells, mitogen-activated B cells and Epstein-Barr virus (EBV)-transformed B-cell lines to stimulate the activation and proliferation of allogeneic T cells under a variety of experimental conditions. It was found that resting B cells presented antigen poorly, while activated cells were highly immunogenic. Paraformaldehyde fixation completely eliminated antigen presentation by resting B cells, despite constitutive expression of class II MHC antigens. However, fixation had little effect on antigen presentation by activated B cells that expressed B7-1 and B7-2 in addition to class II major histocompatibility complex (MHC) molecules. Arrest of B-cell activation by serial fixation after treatment with F(ab')2 fragments of goat anti-human IgM produced cells with variable antigen-presenting capacity. Optimal antigen presentation was observed for cells fixed 72 hr after the initiation of B-cell activation. Although both B7-1 and B7-2 antigen expression increased after B-cell activation, it was found that the rate of T-cell proliferation correlated most closely with B7-2 expression. Stimulation of T cells by fixed activated B lymphocytes could be blocked by antibodies directed at class II MHC molecules, indicating involvement of the T-cell antigen receptor. In addition, T-cell proliferation was inhibited by antibodies specific for B7-1 and B7-2 and by the fusion protein CTLA4-Ig, demonstrating a requirement for CD28 signal transduction. The sole requirement of B7 family expression for antigen presentation by B lymphocytes was shown by demonstration of T-cell stimulation by fixed resting B cells in the presence of CD28 antibody as a source of artificial costimulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boussiotis V. A., Freeman G. J., Gribben J. G., Daley J., Gray G., Nadler L. M. Activated human B lymphocytes express three CTLA-4 counterreceptors that costimulate T-cell activation. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11059–11063. doi: 10.1073/pnas.90.23.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R. H., Fearon D. T. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992 Apr 3;256(5053):105–107. doi: 10.1126/science.1373518. [DOI] [PubMed] [Google Scholar]
- Cassell D. J., Schwartz R. H. A quantitative analysis of antigen-presenting cell function: activated B cells stimulate naive CD4 T cells but are inferior to dendritic cells in providing costimulation. J Exp Med. 1994 Nov 1;180(5):1829–1840. doi: 10.1084/jem.180.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut R. W., Colon S. M., Grey H. M. Antigen presentation by normal B cells, B cell tumors, and macrophages: functional and biochemical comparison. J Immunol. 1982 Apr;128(4):1764–1768. [PubMed] [Google Scholar]
- Eynon E. E., Parker D. C. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992 Jan 1;175(1):131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman A. S., Freeman G., Horowitz J. C., Daley J., Nadler L. M. B7, a B-cell-restricted antigen that identifies preactivated B cells. J Immunol. 1987 Nov 15;139(10):3260–3267. [PubMed] [Google Scholar]
- Freeman G. J., Borriello F., Hodes R. J., Reiser H., Gribben J. G., Ng J. W., Kim J., Goldberg J. M., Hathcock K., Laszlo G. Murine B7-2, an alternative CTLA4 counter-receptor that costimulates T cell proliferation and interleukin 2 production. J Exp Med. 1993 Dec 1;178(6):2185–2192. doi: 10.1084/jem.178.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G. J., Borriello F., Hodes R. J., Reiser H., Hathcock K. S., Laszlo G., McKnight A. J., Kim J., Du L., Lombard D. B. Uncovering of functional alternative CTLA-4 counter-receptor in B7-deficient mice. Science. 1993 Nov 5;262(5135):907–909. doi: 10.1126/science.7694362. [DOI] [PubMed] [Google Scholar]
- Freeman G. J., Gribben J. G., Boussiotis V. A., Ng J. W., Restivo V. A., Jr, Lombard L. A., Gray G. S., Nadler L. M. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993 Nov 5;262(5135):909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- Fuchs E. J., Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992 Nov 13;258(5085):1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- Funderud S., Erikstein B., Asheim H. C., Nustad K., Stokke T., Blomhoff H. K., Holte H., Smeland E. B. Functional properties of CD19+ B lymphocytes positively selected from buffy coats by immunomagnetic separation. Eur J Immunol. 1990 Jan;20(1):201–206. doi: 10.1002/eji.1830200129. [DOI] [PubMed] [Google Scholar]
- Harper K., Balzano C., Rouvier E., Mattéi M. G., Luciani M. F., Golstein P. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J Immunol. 1991 Aug 1;147(3):1037–1044. [PubMed] [Google Scholar]
- Hathcock K. S., Laszlo G., Pucillo C., Linsley P., Hodes R. J. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994 Aug 1;180(2):631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho W. Y., Cooke M. P., Goodnow C. C., Davis M. M. Resting and anergic B cells are defective in CD28-dependent costimulation of naive CD4+ T cells. J Exp Med. 1994 May 1;179(5):1539–1549. doi: 10.1084/jem.179.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K., Ashwell J. D., Schwartz R. H. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol. 1988 May 15;140(10):3324–3330. [PubMed] [Google Scholar]
- Jenkins M. K., Taylor P. S., Norton S. D., Urdahl K. B. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991 Oct 15;147(8):2461–2466. [PubMed] [Google Scholar]
- Kakiuchi T., Chesnut R. W., Grey H. M. B cells as antigen-presenting cells: the requirement for B cell activation. J Immunol. 1983 Jul;131(1):109–114. [PubMed] [Google Scholar]
- Krieger J. I., Chesnut R. W., Grey H. M. Capacity of B cells to function as stimulators of a primary mixed leukocyte reaction. J Immunol. 1986 Nov 15;137(10):3117–3123. [PubMed] [Google Scholar]
- Krieger J. I., Grammer S. F., Grey H. M., Chesnut R. W. Antigen presentation by splenic B cells: resting B cells are ineffective, whereas activated B cells are effective accessory cells for T cell responses. J Immunol. 1985 Nov;135(5):2937–2945. [PubMed] [Google Scholar]
- Lafferty K. J., Cunningham A. J. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975 Feb;53(1):27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- Lenschow D. J., Sperling A. I., Cooke M. P., Freeman G., Rhee L., Decker D. C., Gray G., Nadler L. M., Goodnow C. C., Bluestone J. A. Differential up-regulation of the B7-1 and B7-2 costimulatory molecules after Ig receptor engagement by antigen. J Immunol. 1994 Sep 1;153(5):1990–1997. [PubMed] [Google Scholar]
- Lindstein T., June C. H., Ledbetter J. A., Stella G., Thompson C. B. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989 Apr 21;244(4902):339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Urnes M., Grosmaire L. S., Damle N. K., Ledbetter J. A. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991 Sep 1;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Greene J. L., Tan P., Bradshaw J., Ledbetter J. A., Anasetti C., Damle N. K. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992 Dec 1;176(6):1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Ledbetter J. A. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- Liu Y., Janeway C. A., Jr Cells that present both specific ligand and costimulatory activity are the most efficient inducers of clonal expansion of normal CD4 T cells. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3845–3849. doi: 10.1073/pnas.89.9.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. J., Ledbetter J. A., Morishita Y., June C. H., Beatty P. G., Hansen J. A. A 44 kilodalton cell surface homodimer regulates interleukin 2 production by activated human T lymphocytes. J Immunol. 1986 May 1;136(9):3282–3287. [PubMed] [Google Scholar]
- Metlay J. P., Puré E., Steinman R. M. Distinct features of dendritic cells and anti-Ig activated B cells as stimulators of the primary mixed leukocyte reaction. J Exp Med. 1989 Jan 1;169(1):239–254. doi: 10.1084/jem.169.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi N., Freeman G. J., Gault A., Godfrey D., Nadler L. M., Glimcher L. H. Signalling through the MHC class II cytoplasmic domain is required for antigen presentation and induces B7 expression. Nature. 1992 Nov 19;360(6401):266–268. doi: 10.1038/360266a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen A. M., Smeland E. B., Erikstein B. K., Caignault L., Funderud S. A new method for detachment of Dynabeads from positively selected B lymphocytes. J Immunol Methods. 1992 Feb 5;146(2):195–202. doi: 10.1016/0022-1759(92)90228-l. [DOI] [PubMed] [Google Scholar]
- Sieckmann D. G., Asofsky R., Mosier D. E., Zitron I. M., Paul W. E. Activation of mouse lymphocytes by anti-immunoglobulin. I. Parameters of the proliferative response. J Exp Med. 1978 Mar 1;147(3):814–829. doi: 10.1084/jem.147.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vie H., Miller R. A. Estimation by limiting dilution analysis of human IL 2-secreting T cells: detection of IL 2 produced by single lymphokine-secreting T cells. J Immunol. 1986 May 1;136(9):3292–3297. [PubMed] [Google Scholar]
- Watanabe T., Yoshizaki K., Yagura T., Yamamura Y. In vitro antibody formation by human tonsil lymphocytes. J Immunol. 1974 Aug;113(2):608–616. [PubMed] [Google Scholar]
- Yokochi T., Holly R. D., Clark E. A. B lymphoblast antigen (BB-1) expressed on Epstein-Barr virus-activated B cell blasts, B lymphoblastoid cell lines, and Burkitt's lymphomas. J Immunol. 1982 Feb;128(2):823–827. [PubMed] [Google Scholar]
- Yokochi T., Holly R. D., Clark E. A. B lymphoblast antigen (BB-1) expressed on Epstein-Barr virus-activated B cell blasts, B lymphoblastoid cell lines, and Burkitt's lymphomas. J Immunol. 1982 Feb;128(2):823–827. [PubMed] [Google Scholar]


