Abstract
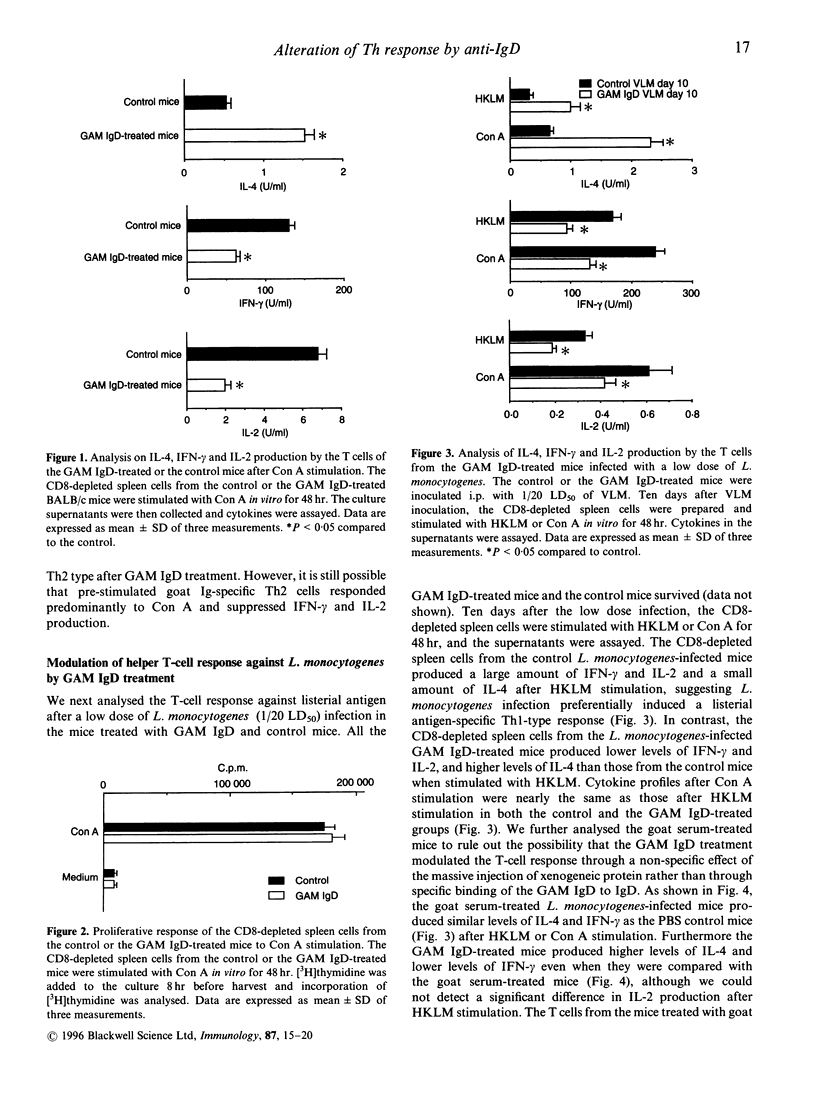
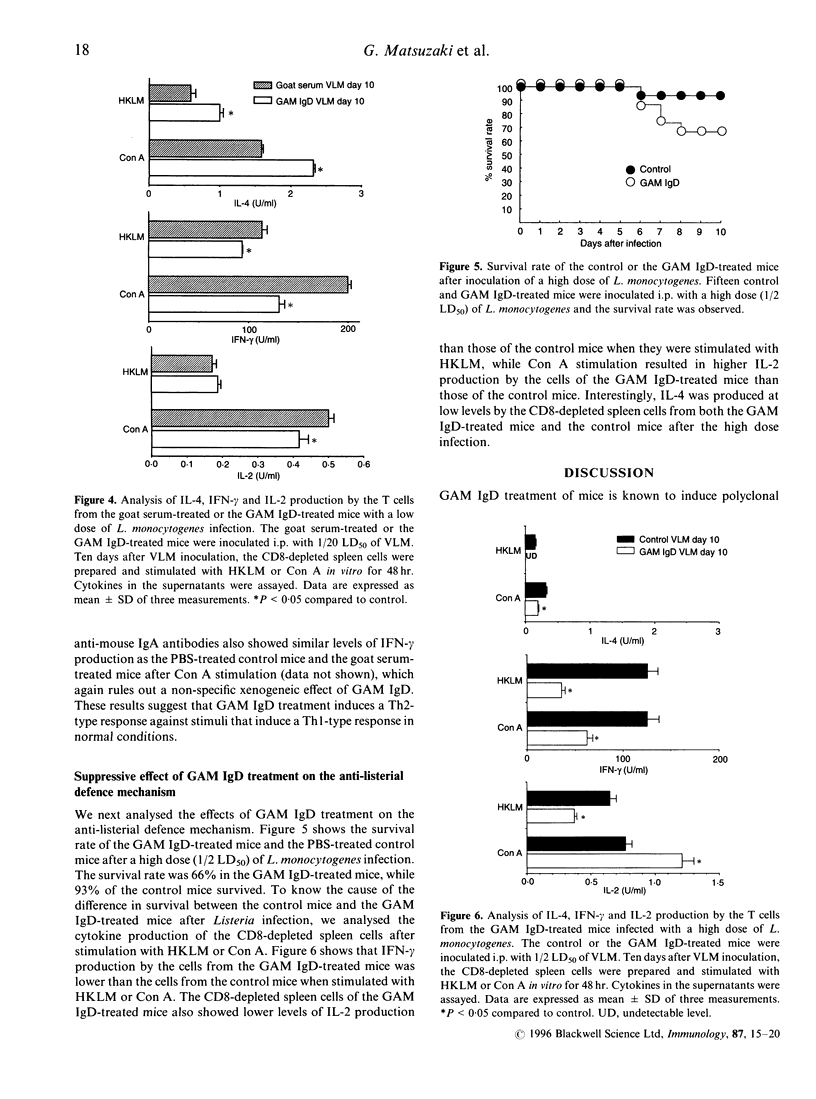
Injection of goat anti-mouse IgD antibodies (GAM IgD) to mice has been shown to induce polyclonal IgG1 and IgE production by B cells and interleukin-4 (IL-4) production by goat Ig-specific T cells. Surface IgD cross-linking also activates B cells to function as antigen-presenting cells (APC). Although the GAM IgD treatment is a well-established system for analysis of B-cell dependent antigen presentation, the influence of GAM IgD treatment on the immune response to irrelevant antigens is not known. To address this issue, we analysed effects of GAM IgD treatment on (1) the mitogen response of freshly isolated T cells, and (2) the listerial antigen-specific response after immunization with viable Listeria monocytogenes, which induces CD4+ interferon-gamma (IFN-gamma) producing protective T cells in normal mice. Spleen CD4+ T cells from the GAM IgD-treated mice produced higher levels of IL-4 but lower levels of IFN-gamma and IL-2 than those from the control mice when they were stimulated with concanavalin A (Con A) in vitro. When spleen T cells were stimulated with listerial antigen 10 days after a low dose (1/20 LD50) of L. monocytogenes infection, CD4+ T cells from the GAM IgD-treated mice showed increased IL-4 production and decreased IFN-gamma and IL-2 production compared with those from the control L. monocytogenes-infected mice. Furthermore, the GAM IgD treatment resulted in a reduction of the survival rate after a high dose (1/2 LD50) of L. monocytogenes infection. These results suggest that treatment of mice with GAM IgD suppresses the T-helper type-1 (Th1)-type T-cell response and induces a Th2-type response against irrelevant antigens, even when they are injected after GAM IgD treatment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. K., Hinrichs D. J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987 Sep 15;139(6):2005–2009. [PubMed] [Google Scholar]
- D'Andrea A., Aste-Amezaga M., Valiante N. M., Ma X., Kubin M., Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993 Sep 1;178(3):1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Ohara J., Goroff D. K., Smith J., Villacreses N., Mond J. J., Paul W. E. Production of BSF-1 during an in vivo, T-dependent immune response. J Immunol. 1986 Nov 1;137(9):2878–2885. [PubMed] [Google Scholar]
- Finkelman F. D., Scher I., Mond J. J., Kessler S., Kung J. T., Metcalf E. S. Polyclonal activation of the murine immune system by an antibody to IgD. II. Generation of polyclonal antibody production and cells with surface IgG. J Immunol. 1982 Aug;129(2):638–646. [PubMed] [Google Scholar]
- Finkelman F. D., Snapper C. M., Mountz J. D., Katona I. M. Polyclonal activation of the murine immune system by a goat antibody to mouse IgD. IX. Induction of a polyclonal IgE response. J Immunol. 1987 May 1;138(9):2826–2830. [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Goroff D. K., Holmes J. M., Bazin H., Nisol F., Finkelman F. D. Polyclonal activation of the murine immune system by an antibody to IgD. XI. Contribution of membrane IgD cross-linking to the generation of an in vivo polyclonal antibody response. J Immunol. 1991 Jan 1;146(1):18–25. [PubMed] [Google Scholar]
- Handa T., Mitsuyama M., Serushago B. A., Muramori K., Nomoto K. Co-operative effect of MCF and MAF(IFN-gamma) in the protection of mice against Listeria monocytogenes. Immunology. 1988 Nov;65(3):427–432. [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. S., Heimberger A. B., Gold J. S., O'Garra A., Murphy K. M. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Huang S., Hendriks W., Althage A., Hemmi S., Bluethmann H., Kamijo R., Vilcek J., Zinkernagel R. M., Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993 Mar 19;259(5102):1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Kamogawa Y., Minasi L. A., Carding S. R., Bottomly K., Flavell R. A. The relationship of IL-4- and IFN gamma-producing T cells studied by lineage ablation of IL-4-producing cells. Cell. 1993 Dec 3;75(5):985–995. doi: 10.1016/0092-8674(93)90542-x. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., Müller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect Immun. 1985 Apr;48(1):263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Mitsuyama M., Handa T., Yayama T., Muramori K., Nomoto K. Induction by killed Listeria monocytogenes of effector T cells mediating delayed-type hypersensitivity but not protection in mice. Immunology. 1987 Oct;62(2):241–248. [PMC free article] [PubMed] [Google Scholar]
- Kricek F., Ruf C., Zunić M., De Jong G., Dukor P., Bahr G. M. Induction in mice of serum IgE levels after treatment with anti-mouse IgD antibodies is preceded by differential modulation of tissue cytokine gene transcription. Eur J Immunol. 1995 Apr;25(4):936–941. doi: 10.1002/eji.1830250412. [DOI] [PubMed] [Google Scholar]
- Morris S. C., Lees A., Finkelman F. D. In vivo activation of naive T cells by antigen-presenting B cells. J Immunol. 1994 Apr 15;152(8):3777–3785. [PubMed] [Google Scholar]
- Morris S. C., Madden K. B., Adamovicz J. J., Gause W. C., Hubbard B. R., Gately M. K., Finkelman F. D. Effects of IL-12 on in vivo cytokine gene expression and Ig isotype selection. J Immunol. 1994 Feb 1;152(3):1047–1056. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Moore K. W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991 Mar;12(3):A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Mieno M., Udono H., Yamaguchi K., Usui T., Hara K., Shiku H., Nakayama E. Roles of CD4+ and CD8+ cells, and the effect of administration of recombinant murine interferon gamma in listerial infection. J Exp Med. 1990 Apr 1;171(4):1141–1154. doi: 10.1084/jem.171.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F., Matsuzaki G., Mitsuyama M., Nomoto K. In vitro generation of IFN-gamma-producing Listeria-specific T cells is dependent on IFN-gamma production by non-NK cells. Cell Immunol. 1995 Feb;160(2):211–216. doi: 10.1016/0008-8749(95)80030-m. [DOI] [PubMed] [Google Scholar]
- Svetić A., Finkelman F. D., Jian Y. C., Dieffenbach C. W., Scott D. E., McCarthy K. F., Steinberg A. D., Gause W. C. Cytokine gene expression after in vivo primary immunization with goat antibody to mouse IgD antibody. J Immunol. 1991 Oct 1;147(7):2391–2397. [PubMed] [Google Scholar]
- Tanaka T., Hu-Li J., Seder R. A., Fazekas de St Groth B., Paul W. E. Interleukin 4 suppresses interleukin 2 and interferon gamma production by naive T cells stimulated by accessory cell-dependent receptor engagement. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5914–5918. doi: 10.1073/pnas.90.13.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]


