Abstract
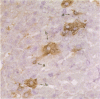
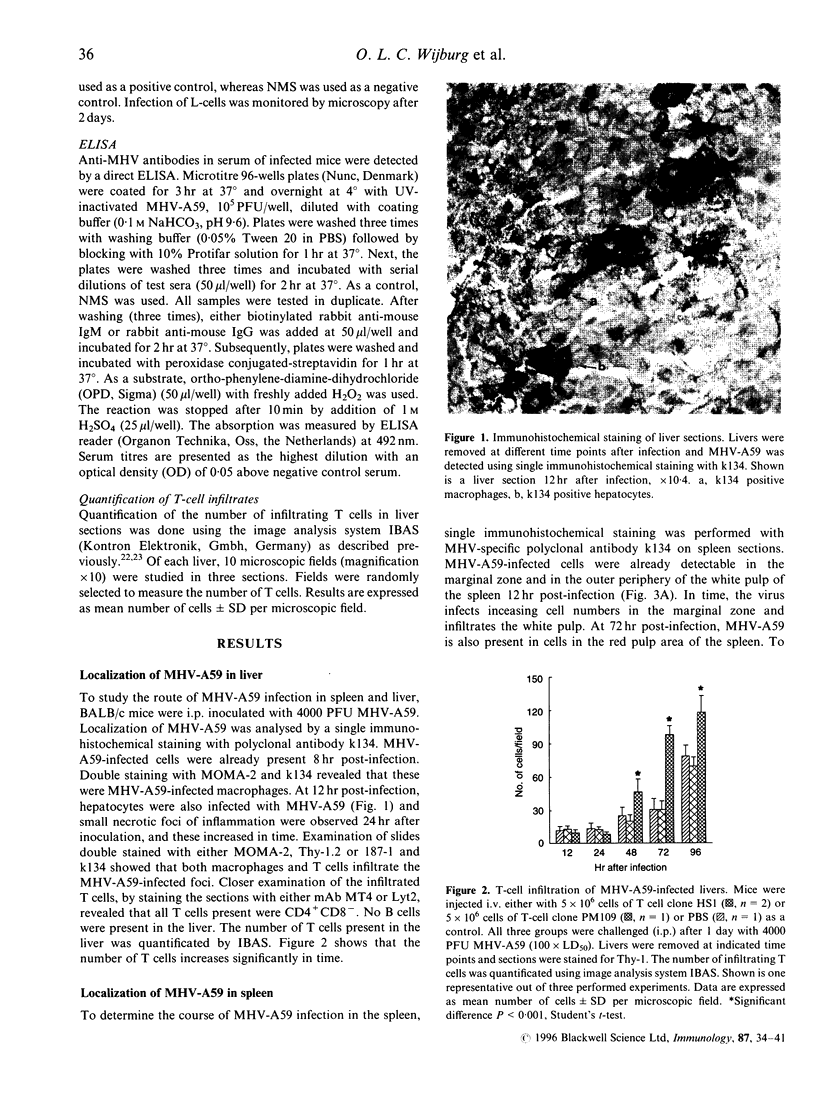
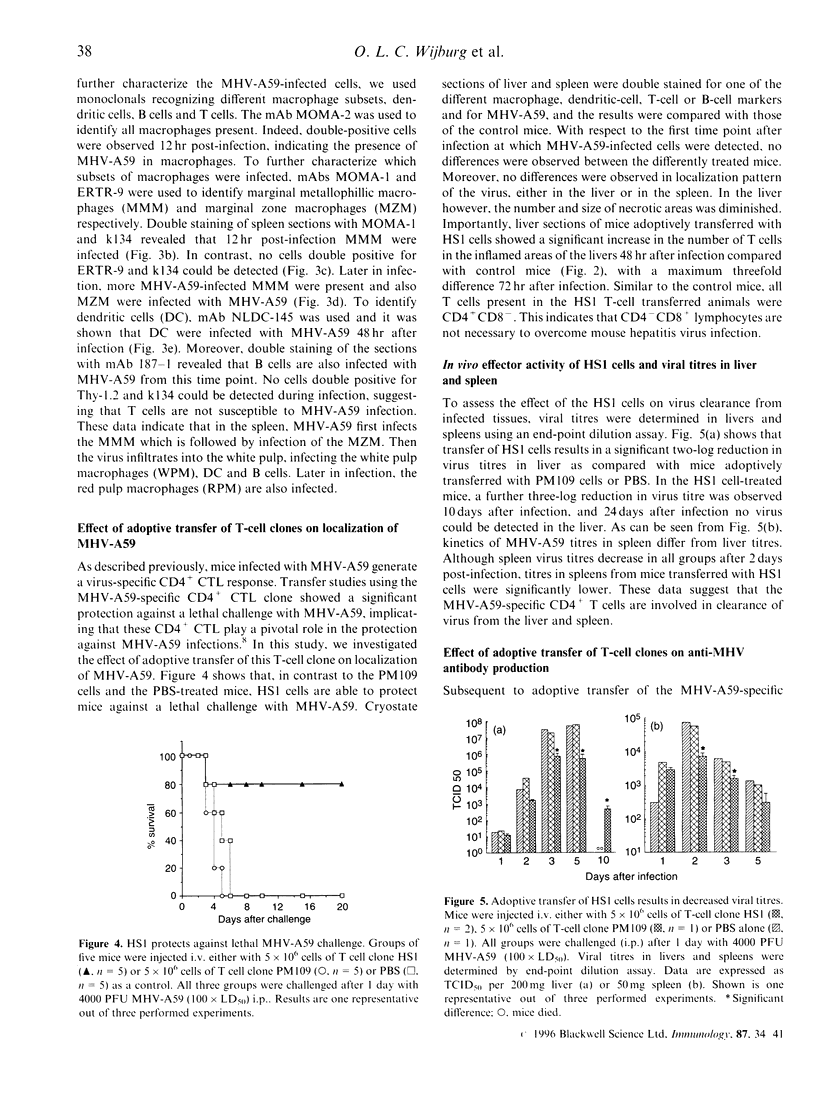
Macrophages and T lymphocytes play an important role in recovery from viral infections. During mouse hepatitis virus (MHV-A59) infection, a clear virus-specific class II-restricted cytotoxic T-cell response is generated. Transfer of these CD4+ cytotoxic T cells (CTL) into naive mice protects against a lethal challenge with MHV. However, their in vivo antiviral effector mechanism is not yet clear. To further investigate a possible effector mechanism, we studied the effect of adoptive transfer of CD4+ CTL on virus localization in spleen and liver. We showed that adoptive transfer of virus-specific T cells does not affect localization of MHV-A59 in different macrophage subsets. Interestingly, a rapid and large infiltrate of CD4+ T cells in and around MHV-A59-infected foci in the liver was observed early in infection, whereas no CD8+ T cells were detectable. Moreover, transfer of virus-specific T cells resulted in significantly decreased viral titres in the liver and spleen and a marginally increased anti-MHV-A59 IgM production. These results imply an important role for virus-specific CD4+ CTL in elimination of infectious MHV-A59 and induction of an effective immune response in the absence of CD8+ CTL.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askonas B. A., Taylor P. M., Esquivel F. Cytotoxic T cells in influenza infection. Ann N Y Acad Sci. 1988;532:230–237. doi: 10.1111/j.1749-6632.1988.tb36342.x. [DOI] [PubMed] [Google Scholar]
- BURSTONE M. S. Histochemical comparison of naphthol AS-phosphates for the demonstration of phosphatases. J Natl Cancer Inst. 1958 Mar;20(3):601–615. [PubMed] [Google Scholar]
- Binder M., Dolezal I., Wolff K., Pehamberger H. Stereologic estimation of volume-weighted mean nuclear volume as a predictor of prognosis in "thin" malignant melanoma. J Invest Dermatol. 1992 Aug;99(2):180–183. doi: 10.1111/1523-1747.ep12616803. [DOI] [PubMed] [Google Scholar]
- Chung S., Gorczynski R., Cruz B., Fingerote R., Skamene E., Perlman S., Leibowitz J., Fung L., Flowers M., Levy G. A Th1 cell line (3E9.1) from resistant A/J mice inhibits induction of macrophage procoagulant activity in vitro and protects against MHV-3 mortality in vivo. Immunology. 1994 Nov;83(3):353–361. [PMC free article] [PubMed] [Google Scholar]
- Claassen E., Boorsma D. M., Kors N., Van Rooijen N. Double-enzyme conjugates, producing an intermediate color, for simultaneous and direct detection of three different intracellular immunoglobulin determinants with only two enzymes. J Histochem Cytochem. 1986 Apr;34(4):423–428. doi: 10.1177/34.4.2419394. [DOI] [PubMed] [Google Scholar]
- Coutelier J. P., Godfraind C., Dveksler G. S., Wysocka M., Cardellichio C. B., Noël H., Holmes K. V. B lymphocyte and macrophage expression of carcinoembryonic antigen-related adhesion molecules that serve as receptors for murine coronavirus. Eur J Immunol. 1994 Jun;24(6):1383–1390. doi: 10.1002/eji.1830240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Allan W., Eichelberger M., Carding S. R. Roles of alpha beta and gamma delta T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- Finke D., Liebert U. G. CD4+ T cells are essential in overcoming experimental murine measles encephalitis. Immunology. 1994 Oct;83(2):184–189. [PMC free article] [PubMed] [Google Scholar]
- Graham M. B., Braciale V. L., Braciale T. J. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994 Oct 1;180(4):1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M. B., Dalton D. K., Giltinan D., Braciale V. L., Stewart T. A., Braciale T. J. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J Exp Med. 1993 Nov 1;178(5):1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk M. H., Schoemaker H. M., De Jong I., Schijns V. E., Spaan W. J., Boog C. J. Differential activation of mouse hepatitis virus-specific CD4+ cytotoxic T cells is defined by peptide length. Immunology. 1995 Aug;85(4):517–522. [PMC free article] [PubMed] [Google Scholar]
- Heemskerk M. H., Schoemaker H. M., Spaan W. J., Boog C. J. Predominance of MHC class II-restricted CD4+ cytotoxic T cells against mouse hepatitis virus A59. Immunology. 1995 Apr;84(4):521–527. [PMC free article] [PubMed] [Google Scholar]
- Holmes K. V., Welsh R. M., Haspel M. V. Natural cytotoxicity against mouse hepatitis virus-infected target cells. I. Correlation of cytotoxicity with virus binding to leukocytes. J Immunol. 1986 Feb 15;136(4):1446–1453. [PubMed] [Google Scholar]
- Kraal G., Breel M., Janse M., Bruin G. Langerhans' cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986 Apr 1;163(4):981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Janse M. Marginal metallophilic cells of the mouse spleen identified by a monoclonal antibody. Immunology. 1986 Aug;58(4):665–669. [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Rep M., Janse M. Macrophages in T and B cell compartments and other tissue macrophages recognized by monoclonal antibody MOMA-2. An immunohistochemical study. Scand J Immunol. 1987 Dec;26(6):653–661. doi: 10.1111/j.1365-3083.1987.tb02301.x. [DOI] [PubMed] [Google Scholar]
- Körner H., Schliephake A., Winter J., Zimprich F., Lassmann H., Sedgwick J., Siddell S., Wege H. Nucleocapsid or spike protein-specific CD4+ T lymphocytes protect against coronavirus-induced encephalomyelitis in the absence of CD8+ T cells. J Immunol. 1991 Oct 1;147(7):2317–2323. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Palmen M. J., Dieleman L. A., van der Ende M. B., Uyterlinde A., Peña A. S., Meuwissen S. G., van Rees E. P. Non-lymphoid and lymphoid cells in acute, chronic and relapsing experimental colitis. Clin Exp Immunol. 1995 Feb;99(2):226–232. doi: 10.1111/j.1365-2249.1995.tb05537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierres A., Naquet P., Van Agthoven A., Bekkhoucha F., Denizot F., Mishal Z., Schmitt-Verhulst A. M., Pierres M. A rat anti-mouse T4 monoclonal antibody (H129.19) inhibits the proliferation of Ia-reactive T cell clones and delineates two phenotypically distinct (T4+, Lyt-2,3-, and T4-, Lyt-2,3+) subsets among anti-Ia cytolytic T cell clones. J Immunol. 1984 Jun;132(6):2775–2782. [PubMed] [Google Scholar]
- Rottier P. J., Spaan W. J., Horzinek M. C., van der Zeijst B. A. Translation of three mouse hepatitis virus strain A59 subgenomic RNAs in Xenopus laevis oocytes. J Virol. 1981 Apr;38(1):20–26. doi: 10.1128/jvi.38.1.20-26.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seid J. M., Liberto M., Bonina L., Leung K. N., Nash A. A. T cell-macrophage interactions in the immune response to herpes simplex virus: the significance of interferon-gamma. J Gen Virol. 1986 Dec;67(Pt 12):2799–2802. doi: 10.1099/0022-1317-67-12-2799. [DOI] [PubMed] [Google Scholar]
- Spaan W. J., Rottier P. J., Horzinek M. C., van der Zeijst B. A. Isolation and identification of virus-specific mRNAs in cells infected with mouse hepatitis virus (MHV-A59). Virology. 1981 Jan 30;108(2):424–434. doi: 10.1016/0042-6822(81)90449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S. A., Matsushima G. K., Casteel N., Weiner L. P. In vivo effects of coronavirus-specific T cell clones: DTH inducer cells prevent a lethal infection but do not inhibit virus replication. J Immunol. 1986 Apr 15;136(8):3052–3056. [PubMed] [Google Scholar]
- Stout R. D. Macrophage activation by T cells: cognate and non-cognate signals. Curr Opin Immunol. 1993 Jun;5(3):398–403. doi: 10.1016/0952-7915(93)90059-2. [DOI] [PubMed] [Google Scholar]
- Taylor P. M., Esquivel F., Askonas B. A. Murine CD4+ T cell clones vary in function in vitro and in influenza infection in vivo. Int Immunol. 1990;2(4):323–328. doi: 10.1093/intimm/2.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H., Siddell S., ter Meulen V. The biology and pathogenesis of coronaviruses. Curr Top Microbiol Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- Weiner L. P. Pathogenesis of demyelination induced by a mouse hepatitis. Arch Neurol. 1973 May;28(5):298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Haspel M. V., Parker D. C., Holmes K. V. Natural cytotoxicity against mouse hepatitis virus-infected cells. II. A cytotoxic effector cell with a B lymphocyte phenotype. J Immunol. 1986 Feb 15;136(4):1454–1460. [PubMed] [Google Scholar]
- Williamson J. S., Stohlman S. A. Effective clearance of mouse hepatitis virus from the central nervous system requires both CD4+ and CD8+ T cells. J Virol. 1990 Sep;64(9):4589–4592. doi: 10.1128/jvi.64.9.4589-4592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. 1986 Oct 30-Nov 5Nature. 323(6091):819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- van Rooijen N. Macrophages as accessory cells in the in vivo humoral immune response: from processing of particulate antigens to regulation by suppression. Semin Immunol. 1992 Aug;4(4):237–245. [PubMed] [Google Scholar]