Abstract
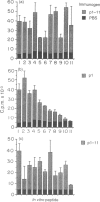
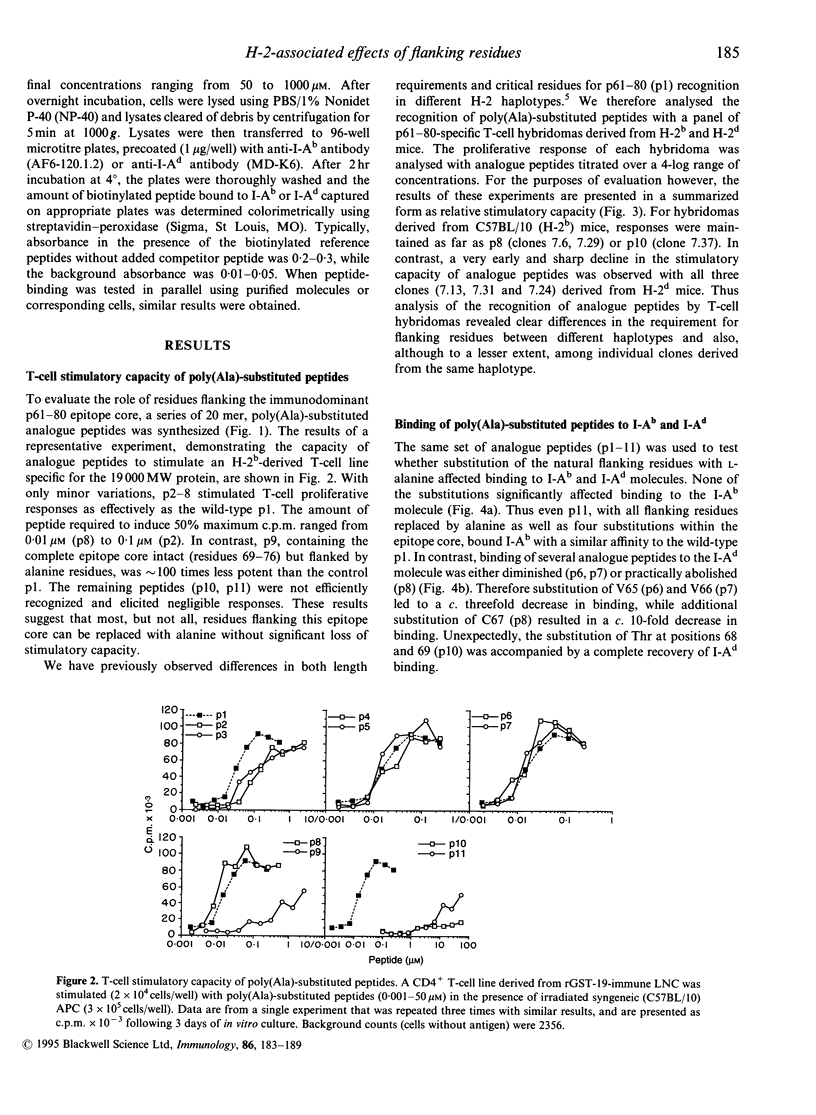
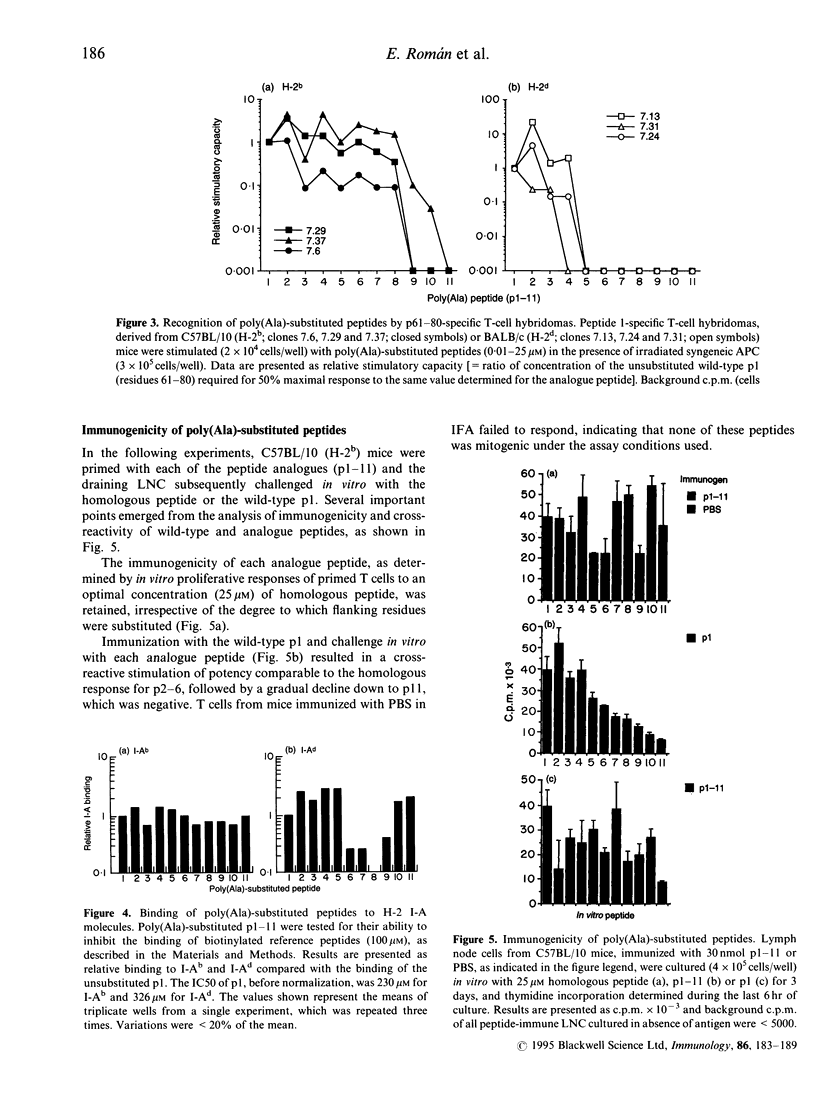
Previously we have identified an immunodominant, eight-residue, epitope core sequence (TAAGNVNI) from the 19,000 MW protein of Mycobacterium tuberculosis, which is recognized in the context of multiple H-2 I-A molecules. In this study, the role of residues flanking this T-cell epitope core was examined, using a series of 20 mer analogue peptides in which the native flanking residues were progressively replaced with L-alanine. Analogue peptides were tested for their capacity to stimulate a CD4+ 19,000 MW protein-specific T-cell line, revealing that all but one N-terminal flanking residue could be replaced collectively by alanine without significant loss of stimulatory activity. However, clear H-2-associated differences in the requirement for flanking residues were demonstrated with peptide-specific T-cell hybridomas. In particular, H-2d-derived hybridomas were much more stringent in their requirement for flanking residues than were H-2b hybridomas. All polyalanine-substituted peptides bound I-Ab molecules, with affinities similar to the native unsubstituted peptide. In contrast, significantly reduced binding to I-Ad was observed with several analogue peptides, although without a clear relationship to the degree of substitution. Furthermore, in H-2b mice, neither immunogenicity nor cross-reactivity with the native peptide showed a clear inverse relationship with respect to the degree of alanine substitution. The results presented in this paper indicate that flanking residues can influence T-cell specificity and that these effects may be controlled by major histocompatibility complex (MHC) haplotype.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Nun A., Choi E., McIntyre K. R., Leeman S. A., McKean D. J., Seidman J. G., Glimcher L. H. DNA-mediated transfer of major histocompatibility class II I-Ab and I-Abm12 genes into B lymphoma cells: molecular and functional analysis of introduced antigens. J Immunol. 1985 Aug;135(2):1456–1464. [PubMed] [Google Scholar]
- Boyer M., Novak Z., Fotedar A., Fraga E., Singh B. Critical role of an amino acid residue in a T cell determinant is due to its interaction with a neighboring non-critical residue. Eur J Immunol. 1990 Sep;20(9):2145–2148. doi: 10.1002/eji.1830200939. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T. S., Gorga J. C., Stern L. J., Urban R. G., Strominger J. L., Wiley D. C. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993 Jul 1;364(6432):33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- Cox J. H., Ivanyi J., Young D. B., Lamb J. R., Syred A. D., Francis M. J. Orientation of epitopes influences the immunogenicity of synthetic peptide dimers. Eur J Immunol. 1988 Dec;18(12):2015–2019. doi: 10.1002/eji.1830181222. [DOI] [PubMed] [Google Scholar]
- Dyrberg T., Oldstone M. B. Peptides as antigens. Importance of orientation. J Exp Med. 1986 Oct 1;164(4):1344–1349. doi: 10.1084/jem.164.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr L. C., Yewdell J. W., Bennink J. R. Flanking sequences influence the presentation of an endogenously synthesized peptide to cytotoxic T lymphocytes. J Exp Med. 1992 Feb 1;175(2):481–487. doi: 10.1084/jem.175.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evavold B. D., Allen P. M. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science. 1991 May 31;252(5010):1308–1310. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]
- Gammon G., Geysen H. M., Apple R. J., Pickett E., Palmer M., Ametani A., Sercarz E. E. T cell determinant structure: cores and determinant envelopes in three mouse major histocompatibility complex haplotypes. J Exp Med. 1991 Mar 1;173(3):609–617. doi: 10.1084/jem.173.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe T., Harris D., Vordermeier M., Lathigra R., Ivanyi J., Young D. Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: immunological analysis and evidence of glycosylation. Infect Immun. 1993 Jan;61(1):260–267. doi: 10.1128/iai.61.1.260-267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam A. M., Pearson C. I., Smilek D. E., Steinman L., McDevitt H. O. A polyalanine peptide with only five native myelin basic protein residues induces autoimmune encephalomyelitis. J Exp Med. 1992 Aug 1;176(2):605–609. doi: 10.1084/jem.176.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer J., Belunis C., Bolin D., Papadopoulos J., Walsky R., Higelin J., Danho W., Sinigaglia F., Nagy Z. A. High-affinity binding of short peptides to major histocompatibility complex class II molecules by anchor combinations. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4456–4460. doi: 10.1073/pnas.91.10.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. P., Vordermeier H. M., Arya A., Moreno C., Ivanyi J. Permissive recognition of a mycobacterial T-cell epitope: localization of overlapping epitope core sequences recognized in association with multiple major histocompatibility complex class II I-A molecules. Immunology. 1995 Apr;84(4):555–561. [PMC free article] [PubMed] [Google Scholar]
- Harris D. P., Vordermeier H. M., Friscia G., Román E., Surcel H. M., Pasvol G., Moreno C., Ivanyi J. Genetically permissive recognition of adjacent epitopes from the 19-kDa antigen of Mycobacterium tuberculosis by human and murine T cells. J Immunol. 1993 Jun 1;150(11):5041–5050. [PubMed] [Google Scholar]
- Janssen R., Wauben M., van der Zee R., de Gast M., Tommassen J. Influence of amino acids of a carrier protein flanking an inserted T cell determinant on T cell stimulation. Int Immunol. 1994 Aug;6(8):1187–1193. doi: 10.1093/intimm/6.8.1187. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Gorga J. C., Busch R., Rothbard J., Strominger J. L., Wiley D. C. Peptide binding to HLA-DR1: a peptide with most residues substituted to alanine retains MHC binding. EMBO J. 1990 Jun;9(6):1797–1803. doi: 10.1002/j.1460-2075.1990.tb08304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc C., Martineau P., Charbit A., Lo-Man R., Dériaud E., Hofnung M. Immunodominance of a recombinant T-cell epitope depends on its molecular environment. Mol Immunol. 1993 Dec;30(17):1561–1572. doi: 10.1016/0161-5890(93)90447-j. [DOI] [PubMed] [Google Scholar]
- Manca F., Habeshaw J. A., Dalgleish A. G., Fenoglio D., Li Pira G., Sercarz E. E. Role of flanking variable sequences in antigenicity of consensus regions of HIV gp120 for recognition by specific human T helper clones. Eur J Immunol. 1993 Jan;23(1):269–274. doi: 10.1002/eji.1830230142. [DOI] [PubMed] [Google Scholar]
- Praud C., Jurcevic S., L'Faqihi F. E., Guiraud M., de Preval C., Thomsen M. Promiscuous and specific binding of HIV peptides to HLA-DR1 and DR103. Impact on T-cell repertoire of nonimmunized individuals. Hum Immunol. 1994 Sep;41(1):56–60. doi: 10.1016/0198-8859(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Gefter M. L. Interactions between immunogenic peptides and MHC proteins. Annu Rev Immunol. 1991;9:527–565. doi: 10.1146/annurev.iy.09.040191.002523. [DOI] [PubMed] [Google Scholar]
- Sette A., Buus S., Colon S., Miles C., Grey H. M. Structural analysis of peptides capable of binding to more than one Ia antigen. J Immunol. 1989 Jan 1;142(1):35–40. [PubMed] [Google Scholar]
- Sette A., Buus S., Colon S., Smith J. A., Miles C., Grey H. M. Structural characteristics of an antigen required for its interaction with Ia and recognition by T cells. 1987 Jul 30-Aug 5Nature. 328(6129):395–399. doi: 10.1038/328395a0. [DOI] [PubMed] [Google Scholar]
- Sloan-Lancaster J., Evavold B. D., Allen P. M. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993 May 13;363(6425):156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- Suhrbier A., Rodda S. J., Ho P. C., Csurhes P., Dunckley H., Saul A., Geysen H. M., Rzepczyk C. M. Role of single amino acids in the recognition of a T cell epitope. J Immunol. 1991 Oct 15;147(8):2507–2513. [PubMed] [Google Scholar]
- Van Noort J. M., Boon J., Van der Drift A. C., Wagenaar J. P., Boots A. M., Boog C. J. Antigen processing by endosomal proteases determines which sites of sperm-whale myoglobin are eventually recognized by T cells. Eur J Immunol. 1991 Sep;21(9):1989–1996. doi: 10.1002/eji.1830210904. [DOI] [PubMed] [Google Scholar]
- Vignali D. A., Strominger J. L. Amino acid residues that flank core peptide epitopes and the extracellular domains of CD4 modulate differential signaling through the T cell receptor. J Exp Med. 1994 Jun 1;179(6):1945–1956. doi: 10.1084/jem.179.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vordermeier H. M., Harris D. P., Moreno C., Ivanyi J. Promiscuous T cell recognition of an H-2 IA-presented mycobacterial epitope. Eur J Immunol. 1994 Sep;24(9):2061–2067. doi: 10.1002/eji.1830240919. [DOI] [PubMed] [Google Scholar]
- Wall K. A., Hu J. Y., Currier P., Southwood S., Sette A., Infante A. J. A disease-related epitope of Torpedo acetylcholine receptor. Residues involved in I-Ab binding, self-nonself discrimination, and TCR antagonism. J Immunol. 1994 May 1;152(9):4526–4536. [PubMed] [Google Scholar]
- Wraith D. C., Bruun B., Fairchild P. J. Cross-reactive antigen recognition by an encephalitogenic T cell receptor. Implications for T cell biology and autoimmunity. J Immunol. 1992 Dec 1;149(11):3765–3770. [PubMed] [Google Scholar]