Abstract
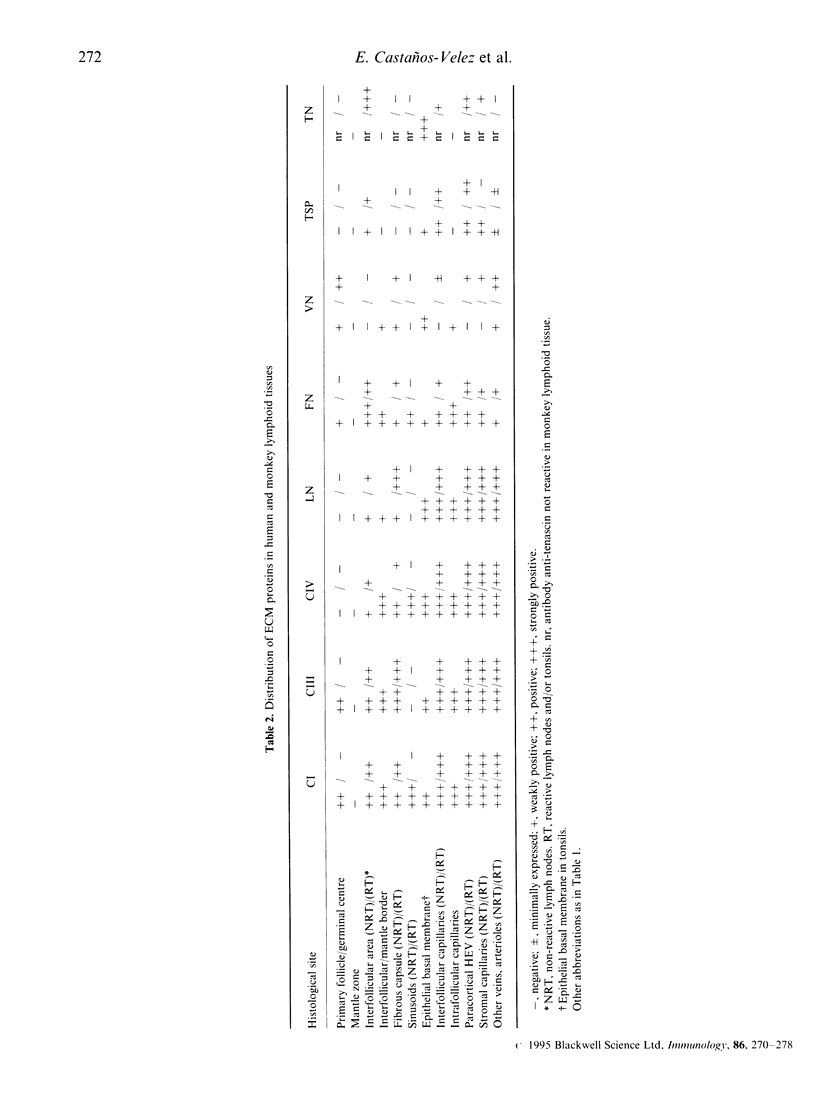
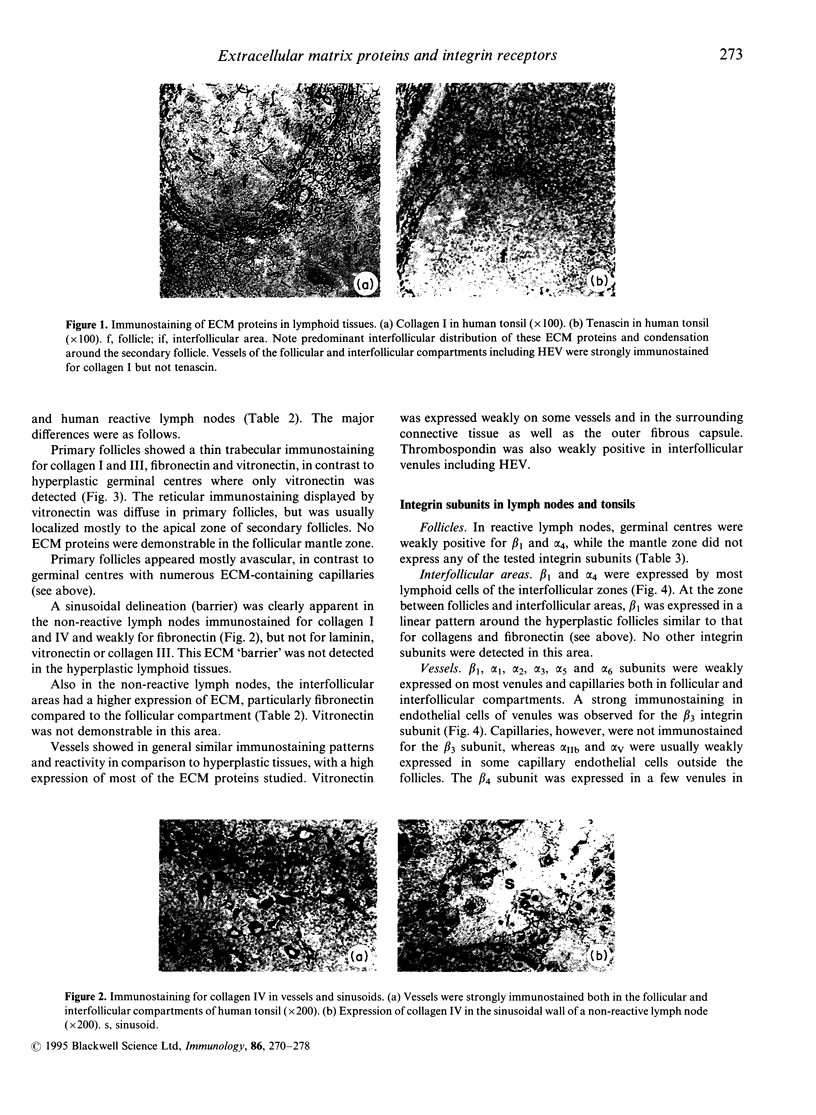
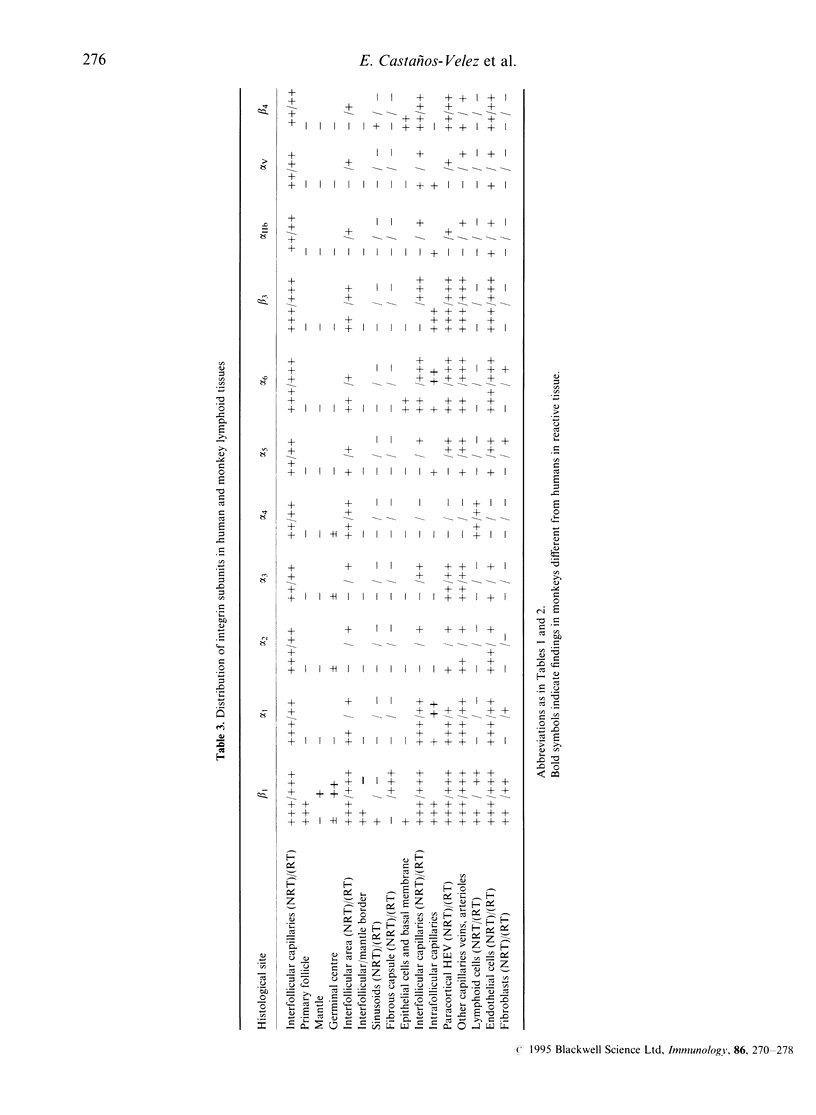
The extracellular matrix (ECM) proteins collagen I, III and IV, laminin, fibronectin, vitronectin, thrombospondin, tenascin and their integrin receptors of the beta 1 and beta 3 subfamilies showed characteristic patterns of distribution in different compartments of non-reactive and reactive lymph nodes (human and monkey). This was particularly evident during development of germinal centres. Thus, ECM proteins (collagens, laminin, fibronectin and tenascin) were abundant in the interfollicular (T-cell rich) compartments of non-reactive as well as reactive lymph nodes. In primary follicles, collagen I, III and fibronectin were expressed but displaced by the expanding germinal centre during the formation of secondary follicles in reactive lymphoid tissues. The integrin subunits were mainly associated with endothelial cells and lymphoid cells in interfollicular areas, but were absent or only poorly expressed in primary as well as secondary follicles. Evidently the expression of ECM components and their integrin receptors is markedly down-regulated in the reactive, highly proliferative germinal centres.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Bourdon M. A., Ruoslahti E. Tenascin mediates cell attachment through an RGD-dependent receptor. J Cell Biol. 1989 Mar;108(3):1149–1155. doi: 10.1083/jcb.108.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi M., Lestani M., Benedetti A., Montagna L., Pedron S., Scarpa A., Menestrina F., Hirohashi S., Pizzolo G., Semenzato G. Constitutive expression of tenascin in T-dependent zones of human lymphoid tissues. Am J Pathol. 1993 Nov;143(5):1348–1355. [PMC free article] [PubMed] [Google Scholar]
- Dameron K. M., Volpert O. V., Tainsky M. A., Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994 Sep 9;265(5178):1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- De Pasquale A., Biagini G., Pileri S., Franchini M., Bottanelli V., Rizzoli R., Vasi V., Castaldini C., Milanesi S. Fibronectin distribution during lymph node development in guinea pig: an immunohistochemical study. Acta Anat (Basel) 1986;126(3):160–162. doi: 10.1159/000146207. [DOI] [PubMed] [Google Scholar]
- De Sousa M., Da Silva M. T., Kupiec-Weglinski J. W. Collagen, the circulation and positioning of lymphocytes: a unifying clue? Scand J Immunol. 1990 Mar;31(3):249–256. doi: 10.1111/j.1365-3083.1990.tb02766.x. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Gloghini A., Carbone A. The nonlymphoid microenvironment of reactive follicles and lymphomas of follicular origin as defined by immunohistology on paraffin-embedded tissues. Hum Pathol. 1993 Jan;24(1):67–76. doi: 10.1016/0046-8177(93)90065-o. [DOI] [PubMed] [Google Scholar]
- Halstensen T. S., Mollnes T. E., Brandtzaeg P. Terminal complement complex (TCC) and S-protein (vitronectin) on follicular dendritic cells in human lymphoid tissues. Immunology. 1988 Oct;65(2):193–197. [PMC free article] [PubMed] [Google Scholar]
- Jones P. L., Schmidhauser C., Bissell M. J. Regulation of gene expression and cell function by extracellular matrix. Crit Rev Eukaryot Gene Expr. 1993;3(2):137–154. [PubMed] [Google Scholar]
- Kaaya E. E., Parravicini C., Sundelin B., Mgaya E., Kitinya J., Lema L., Luande J., Biberfeld P. Spindle cell ploidy and proliferation in endemic and epidemic African Kaposi's sarcoma. Eur J Cancer. 1992;28A(11):1890–1894. doi: 10.1016/0959-8049(92)90030-6. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Rosen S. D., McDonald K. A. Basement-membrane components associated with the extracellular matrix of the lymph node. Cell Tissue Res. 1988 May;252(2):367–375. doi: 10.1007/BF00214379. [DOI] [PubMed] [Google Scholar]
- Lahav J. The functions of thrombospondin and its involvement in physiology and pathophysiology. Biochim Biophys Acta. 1993 Aug 4;1182(1):1–14. doi: 10.1016/0925-4439(93)90146-r. [DOI] [PubMed] [Google Scholar]
- Li S. L., Kaaya E., Feichtinger H., Biberfeld G., Biberfeld P. Immunohistochemical distribution of leucocyte antigens in lymphoid tissues of cynomolgus monkeys (Macaca fascicularis). J Med Primatol. 1993 Jul;22(5):285–293. [PubMed] [Google Scholar]
- Mechtersheimer G., Barth T., Quentmeier A., Möller P. Differential expression of beta 1 integrins in nonneoplastic smooth and striated muscle cells and in tumors derived from these cells. Am J Pathol. 1994 Jun;144(6):1172–1182. [PMC free article] [PubMed] [Google Scholar]
- Möller P., Eichelmann A., Koretz K., Mechtersheimer G. Adhesion molecules VLA-1 to VLA-6 define discrete stages of peripheral B lymphocyte development and characterize different types of B cell neoplasia. Leukemia. 1992 Apr;6(4):256–264. [PubMed] [Google Scholar]
- Reilly J. T., Nash J. R., Mackie M. J., McVerry B. A. Distribution of fibronectin and laminin in normal and pathological lymphoid tissue. J Clin Pathol. 1985 Aug;38(8):849–854. doi: 10.1136/jcp.38.8.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegg C. R., Chiquet-Ehrismann R., Alkan S. S. Tenascin, an extracellular matrix protein, exerts immunomodulatory activities. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7437–7441. doi: 10.1073/pnas.86.19.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriever F., Freedman A. S., Freeman G., Messner E., Lee G., Daley J., Nadler L. M. Isolated human follicular dendritic cells display a unique antigenic phenotype. J Exp Med. 1989 Jun 1;169(6):2043–2058. doi: 10.1084/jem.169.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Shaw S. Lymphocyte interactions with extracellular matrix. FASEB J. 1991 Jun;5(9):2292–2299. doi: 10.1096/fasebj.5.9.1860621. [DOI] [PubMed] [Google Scholar]
- Soini Y., Alavaikko M., Lehto V. P., Virtanen I. Tenascin in reactive lymph nodes and in malignant lymphomas. Pathol Res Pract. 1992 Dec;188(8):1078–1082. doi: 10.1016/S0344-0338(11)81254-9. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Szekanecz Z., Humphries M. J., Ager A. Lymphocyte adhesion to high endothelium is mediated by two beta 1 integrin receptors for fibronectin, alpha 4 beta 1 and alpha 5 beta 1. J Cell Sci. 1992 Apr;101(Pt 4):885–894. doi: 10.1242/jcs.101.4.885. [DOI] [PubMed] [Google Scholar]
- Tooney P. A., Agrez M. V., Burns G. F. A re-examination of the molecular basis of cell movement. Immunol Cell Biol. 1993 Apr;71(Pt 2):131–139. doi: 10.1038/icb.1993.14. [DOI] [PubMed] [Google Scholar]
- Weinacker A., Chen A., Agrez M., Cone R. I., Nishimura S., Wayner E., Pytela R., Sheppard D. Role of the integrin alpha v beta 6 in cell attachment to fibronectin. Heterologous expression of intact and secreted forms of the receptor. J Biol Chem. 1994 Mar 4;269(9):6940–6948. [PubMed] [Google Scholar]
- Wight T. N., Raugi G. J., Mumby S. M., Bornstein P. Light microscopic immunolocation of thrombospondin in human tissues. J Histochem Cytochem. 1985 Apr;33(4):295–302. doi: 10.1177/33.4.3884704. [DOI] [PubMed] [Google Scholar]
- Zutter M. M. Immunolocalization of integrin receptors in normal lymphoid tissues. Blood. 1991 May 15;77(10):2231–2236. [PubMed] [Google Scholar]
- van den Berg T. K., van der Ende M., Döpp E. A., Kraal G., Dijkstra C. D. Localization of beta 1 integrins and their extracellular ligands in human lymphoid tissues. Am J Pathol. 1993 Oct;143(4):1098–1110. [PMC free article] [PubMed] [Google Scholar]
- van der Valk P., Meijer C. J. The histology of reactive lymph nodes. Am J Surg Pathol. 1987 Nov;11(11):866–882. doi: 10.1097/00000478-198711000-00005. [DOI] [PubMed] [Google Scholar]