Abstract
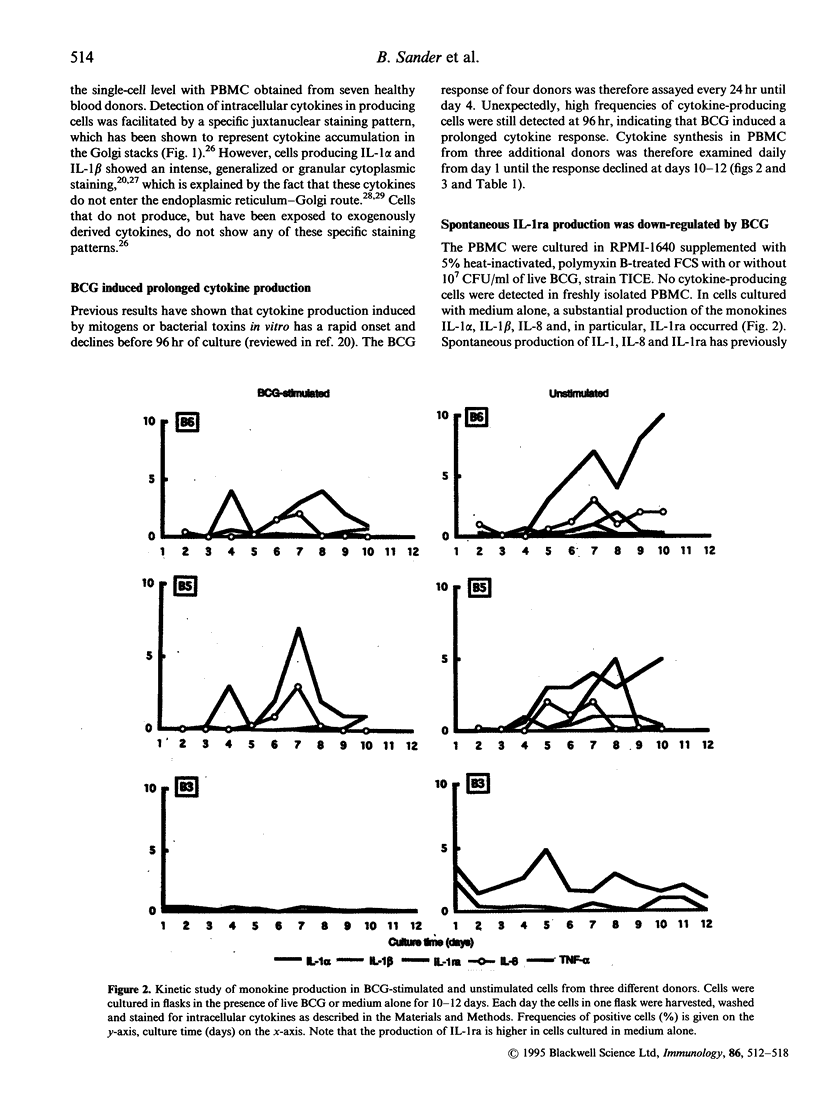
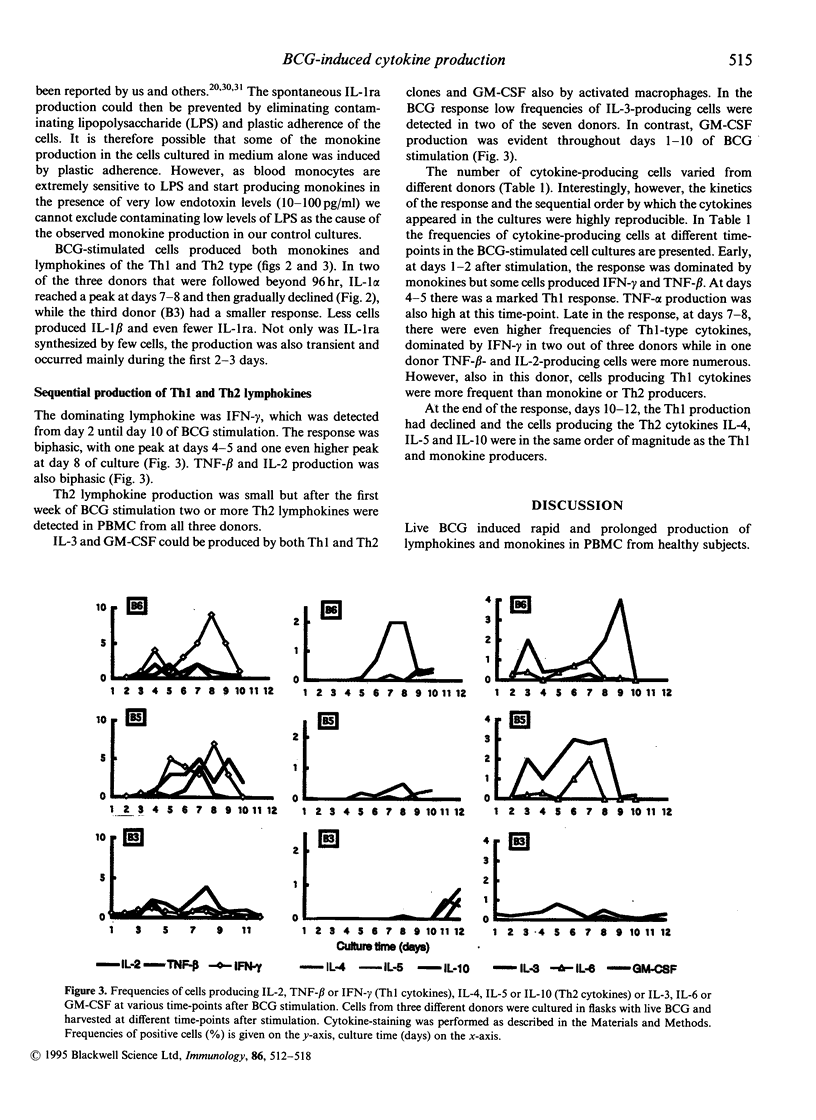
Causes of individual variation in susceptibility to mycobacterial diseases are only partly understood. An efficient cell-mediated immune response is crucial for resistance. Macrophages and T cells interact to eliminate the mycobacteria, partially through the effects of secreted cytokines. A vigorous anti-bacterial inflammatory response is sometimes accompanied by severe tissue damage, while immunosuppression leads to progressive infection. Here, live, attenuated Mycobacterium bovis, bacillus Calmette-Guérin (BCG), was used as a model antigen to study cytokine production at the single-cell level in response to mycobacteria. Peripheral blood mononuclear cells from healthy individuals were challenged in vitro and the kinetics and frequencies of cytokine-producing cells were studied by immunofluorescent visualization of intracellular cytokines. Fourteen cytokines were assayed; interleukin-1 alpha (IL-1 alpha), IL-1 beta, IL-1 receptor antagonist (IL-1ra), IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, interferon-gamma (IFN-gamma), tumour necrosis factor-alpha (TNF-alpha), TNF-beta and granulocyte-macrophage colony-stimulating factor (GM-CSF). A sequential production of T helper-1 (Th1) and T helper-2 (Th2) cytokines was induced by BCG. Early, at days 1-2 after stimulation, the response was dominated by monokines and a low IFN-gamma and TNF-beta production. At days 4-5 there was a marked production of Th1 lymphokines, with approximately 6% IFN-gamma+ cells, 4% TNF-beta+ cells and 2% IL-2+ cells. Late in the reaction, at days 10-12, a Th2 response with IL-4, IL-5 and IL-10 was detected, while the synthesis of Th1 lymphokines and monokines declined. Overall, our results provide further evidence of IFN-gamma as the major cytokine induced by mycobacteria in healthy individuals, but also suggest that Th2 cytokines participate in the response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams J. S., Roncarolo M. G., Yssel H., Andersson U., Gleich G. J., Silver J. E. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992 Jun;127:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Andersson G., Ekre H. P., Alm G., Perlmann P. Monoclonal antibody two-site ELISA for human IFN-gamma. Adaptation for determinations in human serum or plasma. J Immunol Methods. 1989 Dec 20;125(1-2):89–96. doi: 10.1016/0022-1759(89)90081-1. [DOI] [PubMed] [Google Scholar]
- Andersson J., Björk L., Dinarello C. A., Towbin H., Andersson U. Lipopolysaccharide induces human interleukin-1 receptor antagonist and interleukin-1 production in the same cell. Eur J Immunol. 1992 Oct;22(10):2617–2623. doi: 10.1002/eji.1830221022. [DOI] [PubMed] [Google Scholar]
- Andersson J., Nagy S., Björk L., Abrams J., Holm S., Andersson U. Bacterial toxin-induced cytokine production studied at the single-cell level. Immunol Rev. 1992 Jun;127:69–96. doi: 10.1111/j.1600-065x.1992.tb01409.x. [DOI] [PubMed] [Google Scholar]
- Andersson U., Adolf G., Dohlsten M., Möller G., Sjögren H. O. Characterization of individual tumor necrosis factor alpha-and beta-producing cells after polyclonal T cell activation. J Immunol Methods. 1989 Oct 24;123(2):233–240. doi: 10.1016/0022-1759(89)90227-5. [DOI] [PubMed] [Google Scholar]
- Andersson U., Matsuda T. Human interleukin 6 and tumor necrosis factor alpha production studied at a single-cell level. Eur J Immunol. 1989 Jun;19(6):1157–1160. doi: 10.1002/eji.1830190629. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakouche O., Brown D. C., Lachman L. B. Subcellular localization of human monocyte interleukin 1: evidence for an inactive precursor molecule and a possible mechanism for IL 1 release. J Immunol. 1987 Jun 15;138(12):4249–4255. [PubMed] [Google Scholar]
- Chan J., Xing Y., Magliozzo R. S., Bloom B. R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992 Apr 1;175(4):1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. M., Dalton D. K., Stewart T. A., Griffin J. P., Russell D. G., Orme I. M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993 Dec 1;178(6):2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuturi M. C., Murphy M., Costa-Giomi M. P., Weinmann R., Perussia B., Trinchieri G. Independent regulation of tumor necrosis factor and lymphotoxin production by human peripheral blood lymphocytes. J Exp Med. 1987 Jun 1;165(6):1581–1594. doi: 10.1084/jem.165.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton D. K., Pitts-Meek S., Keshav S., Figari I. S., Bradley A., Stewart T. A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993 Mar 19;259(5102):1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Del Prete G. F., De Carli M., Mastromauro C., Biagiotti R., Macchia D., Falagiani P., Ricci M., Romagnani S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991 Jul;88(1):346–350. doi: 10.1172/JCI115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. Growth of Mycobacterium avium in human monocytes: identification of cytokines which reduce and enhance intracellular microbial growth. Eur J Immunol. 1991 Feb;21(2):391–395. doi: 10.1002/eji.1830210221. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Fernandez V., Andersson J., Andersson U., Troye-Blomberg M. Cytokine synthesis analyzed at the single-cell level before and after revaccination with tetanus toxoid. Eur J Immunol. 1994 Aug;24(8):1808–1815. doi: 10.1002/eji.1830240813. [DOI] [PubMed] [Google Scholar]
- Fine P. E. BCG vaccination against tuberculosis and leprosy. Br Med Bull. 1988 Jul;44(3):691–703. doi: 10.1093/oxfordjournals.bmb.a072277. [DOI] [PubMed] [Google Scholar]
- Flynn J. L., Chan J., Triebold K. J., Dalton D. K., Stewart T. A., Bloom B. R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993 Dec 1;178(6):2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T., Andus T., Klapproth J., Hirano T., Kishimoto T., Heinrich P. C. Induction of rat acute-phase proteins by interleukin 6 in vivo. Eur J Immunol. 1988 May;18(5):717–721. doi: 10.1002/eji.1830180510. [DOI] [PubMed] [Google Scholar]
- Haanen J. B., de Waal Malefijt R., Res P. C., Kraakman E. M., Ottenhoff T. H., de Vries R. R., Spits H. Selection of a human T helper type 1-like T cell subset by mycobacteria. J Exp Med. 1991 Sep 1;174(3):583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Pando R., Rook G. A. The role of TNF-alpha in T-cell-mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology. 1994 Aug;82(4):591–595. [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- Lamche H. R., Adolf G. R. Highly sensitive enzyme immunoassays for antibodies to human tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta). J Immunol Methods. 1990 Aug 7;131(2):283–289. doi: 10.1016/0022-1759(90)90200-f. [DOI] [PubMed] [Google Scholar]
- Lamm D. L. Long-term results of intravesical therapy for superficial bladder cancer. Urol Clin North Am. 1992 Aug;19(3):573–580. [PubMed] [Google Scholar]
- Martich G. D., Danner R. L., Ceska M., Suffredini A. F. Detection of interleukin 8 and tumor necrosis factor in normal humans after intravenous endotoxin: the effect of antiinflammatory agents. J Exp Med. 1991 Apr 1;173(4):1021–1024. doi: 10.1084/jem.173.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales A., Eidinger D., Bruce A. W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976 Aug;116(2):180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Moore K. W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991 Mar;12(3):A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- Norrby-Teglund A., Norgren M., Holm S. E., Andersson U., Andersson J. Similar cytokine induction profiles of a novel streptococcal exotoxin, MF, and pyrogenic exotoxins A and B. Infect Immun. 1994 Sep;62(9):3731–3738. doi: 10.1128/iai.62.9.3731-3738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Roberts A. D., Griffin J. P., Abrams J. S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993 Jul 1;151(1):518–525. [PubMed] [Google Scholar]
- Parronchi P., Macchia D., Piccinni M. P., Biswas P., Simonelli C., Maggi E., Ricci M., Ansari A. A., Romagnani S. Allergen- and bacterial antigen-specific T-cell clones established from atopic donors show a different profile of cytokine production. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4538–4542. doi: 10.1073/pnas.88.10.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutsiaka D. D., Clark B. D., Vannier E., Dinarello C. A. Production of interleukin-1 receptor antagonist and interleukin-1 beta by peripheral blood mononuclear cells is differentially regulated. Blood. 1991 Sep 1;78(5):1275–1281. [PubMed] [Google Scholar]
- Rook G. A. Role of activated macrophages in the immunopathology of tuberculosis. Br Med Bull. 1988 Jul;44(3):611–623. doi: 10.1093/oxfordjournals.bmb.a072271. [DOI] [PubMed] [Google Scholar]
- Rubartelli A., Cozzolino F., Talio M., Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990 May;9(5):1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander B., Andersson J., Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991 Feb;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Schurr E., Malo D., Radzioch D., Buschman E., Morgan K., Gros P., Skamene E. Genetic control of innate resistance to mycobacterial infections. Immunol Today. 1991 Mar;12(3):A42–A45. doi: 10.1016/S0167-5699(05)80012-X. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Tsicopoulos A., Hamid Q., Varney V., Ying S., Moqbel R., Durham S. R., Kay A. B. Preferential messenger RNA expression of Th1-type cells (IFN-gamma+, IL-2+) in classical delayed-type (tuberculin) hypersensitivity reactions in human skin. J Immunol. 1992 Apr 1;148(7):2058–2061. [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]