Abstract
Although mice acquire only a slight degree of protection against tuberculosis by immunization with Mycobacterium leprae (M. leprae) hsp65 in incomplete Freund's adjuvant, protection is substantial following immunization by injection with J774 macrophage-like tumour cells that express the protein from the mycobacterial gene via a retroviral vector. We here took the same vector, used it to transfect the gene into normal murine bone marrow cells in vitro, and then used the transfected cells to reconstitute haematopoiesis in lethally irradiated mice. Bone marrow-cell clonal expansion and production of the protein in vivo resulted in specific delayed-type hypersensitivity and protection against challenge with Mycobacterium tuberculosis (M. tuberculosis) in about half of recipients. Counts of live bacteria in liver at 3 weeks were fivefold lower in delayed-type hypersensitivity (DTH)-positive than in DTH-negative mice. Other mice acquired neither DTH nor protection despite the presence of the protein in peripheral blood.
Full text
PDF

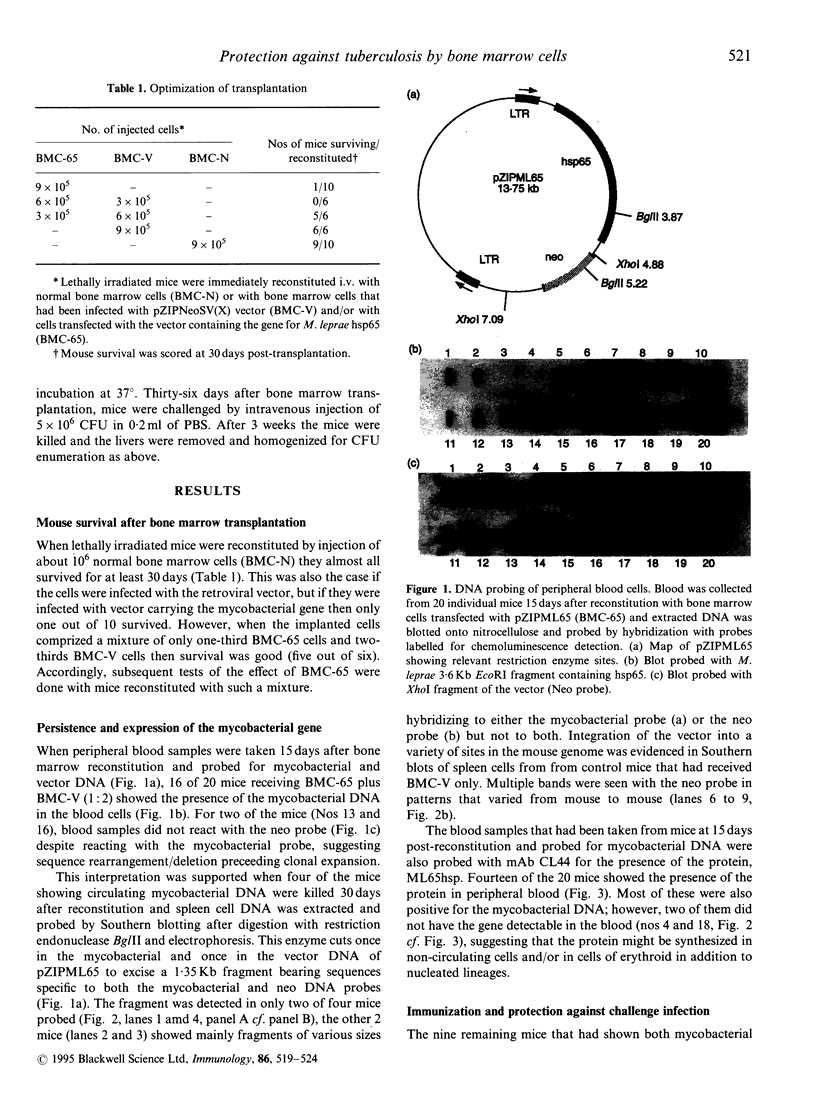
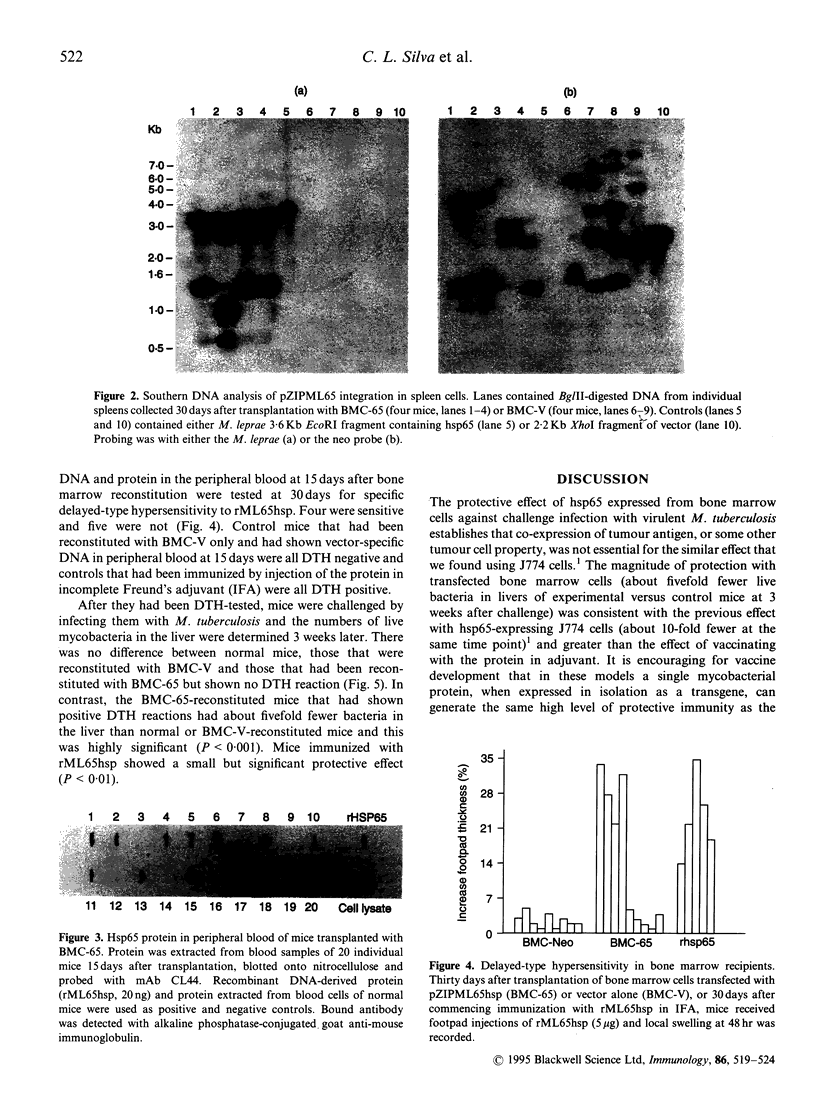
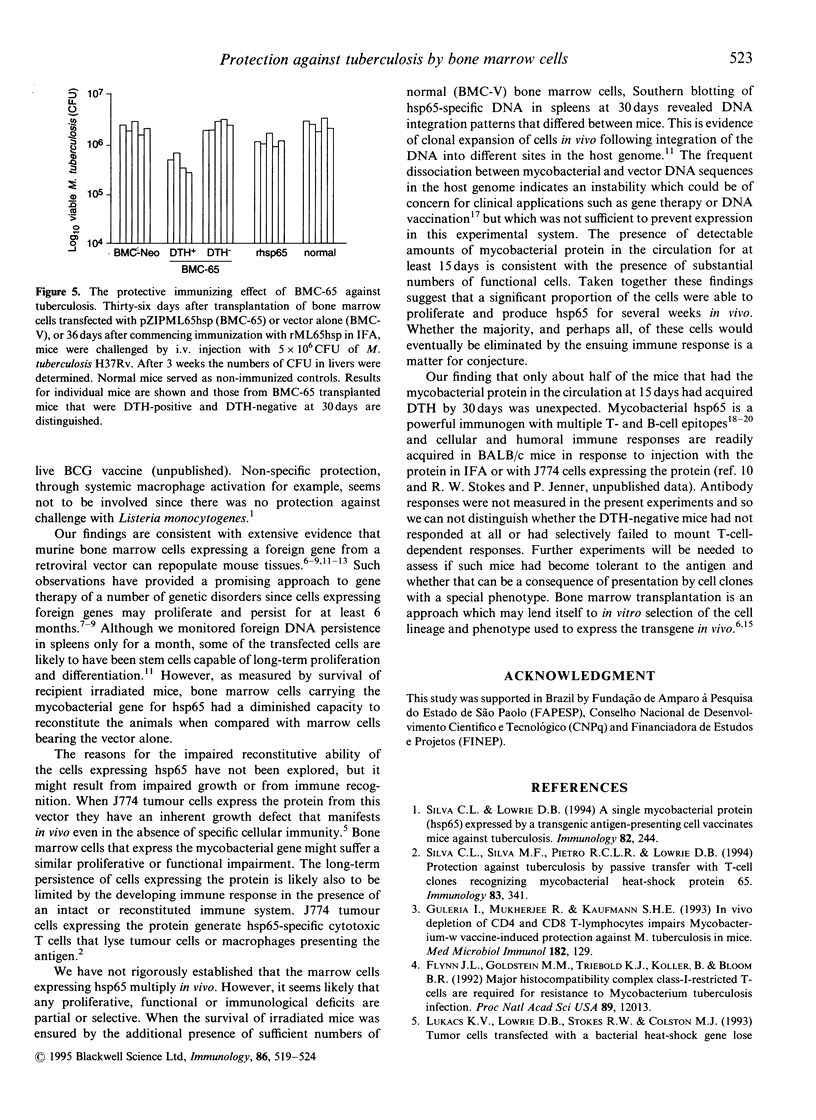

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Barry M. E., Buchanan T. M. Exact definition of species-specific and cross-reactive epitopes of the 65-kilodalton protein of Mycobacterium leprae using synthetic peptides. J Immunol. 1988 Jul 15;141(2):607–613. [PubMed] [Google Scholar]
- Brett S. J., Lamb J. R., Cox J. H., Rothbard J. B., Mehlert A., Ivanyi J. Differential pattern of T cell recognition of the 65-kDa mycobacterial antigen following immunization with the whole protein or peptides. Eur J Immunol. 1989 Jul;19(7):1303–1310. doi: 10.1002/eji.1830190723. [DOI] [PubMed] [Google Scholar]
- Dzierzak E. A., Papayannopoulou T., Mulligan R. C. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature. 1988 Jan 7;331(6151):35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- Flynn J. L., Goldstein M. M., Triebold K. J., Koller B., Bloom B. R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria I., Mukherjee R., Kaufmann S. H. In vivo depletion of CD4 and CD8 T lymphocytes impairs Mycobacterium w vaccine-induced protection against M. tuberculosis in mice. Med Microbiol Immunol. 1993 Jul;182(3):129–135. doi: 10.1007/BF00190265. [DOI] [PubMed] [Google Scholar]
- Krall W. J., Challita P. M., Perlmutter L. S., Skelton D. C., Kohn D. B. Cells expressing human glucocerebrosidase from a retroviral vector repopulate macrophages and central nervous system microglia after murine bone marrow transplantation. Blood. 1994 May 1;83(9):2737–2748. [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Ohashi T., Boggs S., Robbins P., Bahnson A., Patrene K., Wei F. S., Wei J. F., Li J., Lucht L., Fei Y. Efficient transfer and sustained high expression of the human glucocerebrosidase gene in mice and their functional macrophages following transplantation of bone marrow transduced by a retroviral vector. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11332–11336. doi: 10.1073/pnas.89.23.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne W. R., Hock R. A., Kaleko M., Miller A. D. Long-term expression of human adenosine deaminase in mice after transplantation of bone marrow infected with amphotropic retroviral vectors. Hum Gene Ther. 1990 Spring;1(1):31–41. doi: 10.1089/hum.1990.1.1-31. [DOI] [PubMed] [Google Scholar]
- Robertson J. S. Safety considerations for nucleic acid vaccines. Vaccine. 1994 Dec;12(16):1526–1528. doi: 10.1016/0264-410x(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Ross E. A., Anderson N., Micklem H. S. Serial depletion and regeneration of the murine hematopoietic system. Implications for hematopoietic organization and the study of cellular aging. J Exp Med. 1982 Feb 1;155(2):432–444. doi: 10.1084/jem.155.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C. L., Lowrie D. B. A single mycobacterial protein (hsp 65) expressed by a transgenic antigen-presenting cell vaccinates mice against tuberculosis. Immunology. 1994 Jun;82(2):244–248. [PMC free article] [PubMed] [Google Scholar]
- Silva C. L., Silva M. F., Pietro R. C., Lowrie D. B. Protection against tuberculosis by passive transfer with T-cell clones recognizing mycobacterial heat-shock protein 65. Immunology. 1994 Nov;83(3):341–346. [PMC free article] [PubMed] [Google Scholar]
- Spain L. M., Mulligan R. C. Purification and characterization of retrovirally transduced hematopoietic stem cells. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3790–3794. doi: 10.1073/pnas.89.9.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilvassy S. J., Cory S. Efficient retroviral gene transfer to purified long-term repopulating hematopoietic stem cells. Blood. 1994 Jul 1;84(1):74–83. [PubMed] [Google Scholar]
- Van Zant G. Studies of hematopoietic stem cells spared by 5-fluorouracil. J Exp Med. 1984 Mar 1;159(3):679–690. doi: 10.1084/jem.159.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]