Abstract
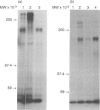
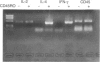
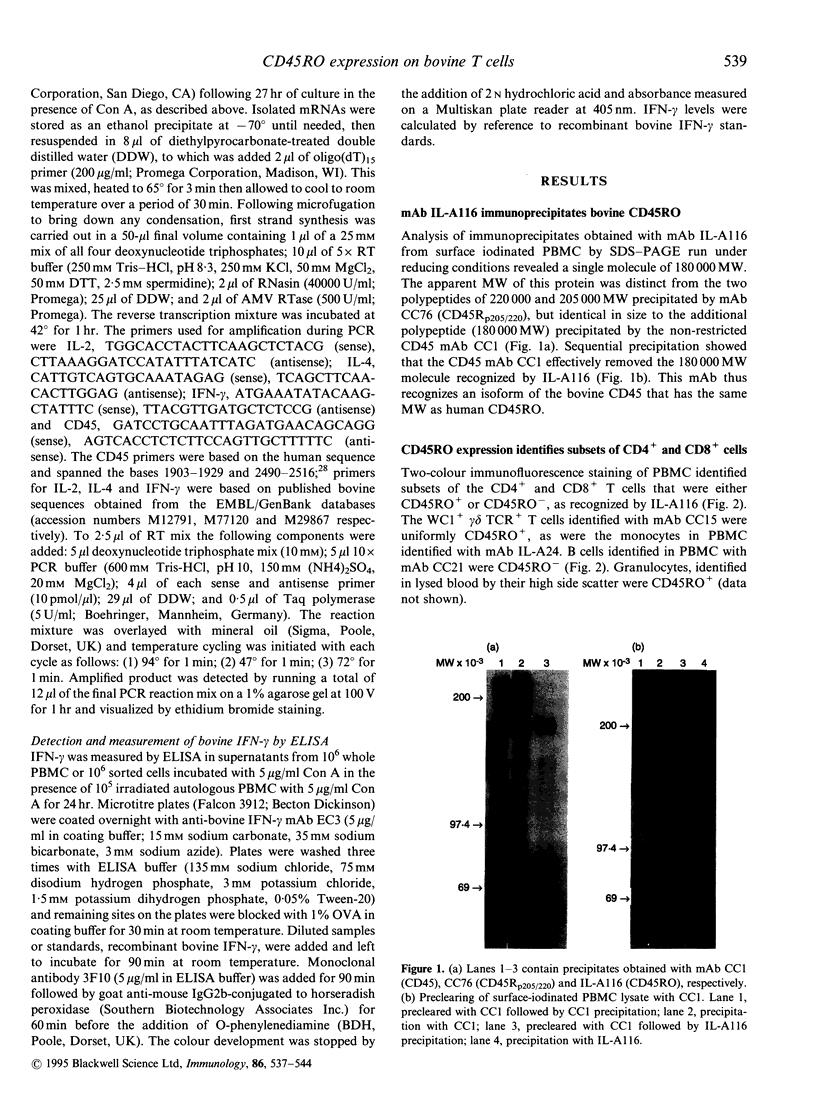
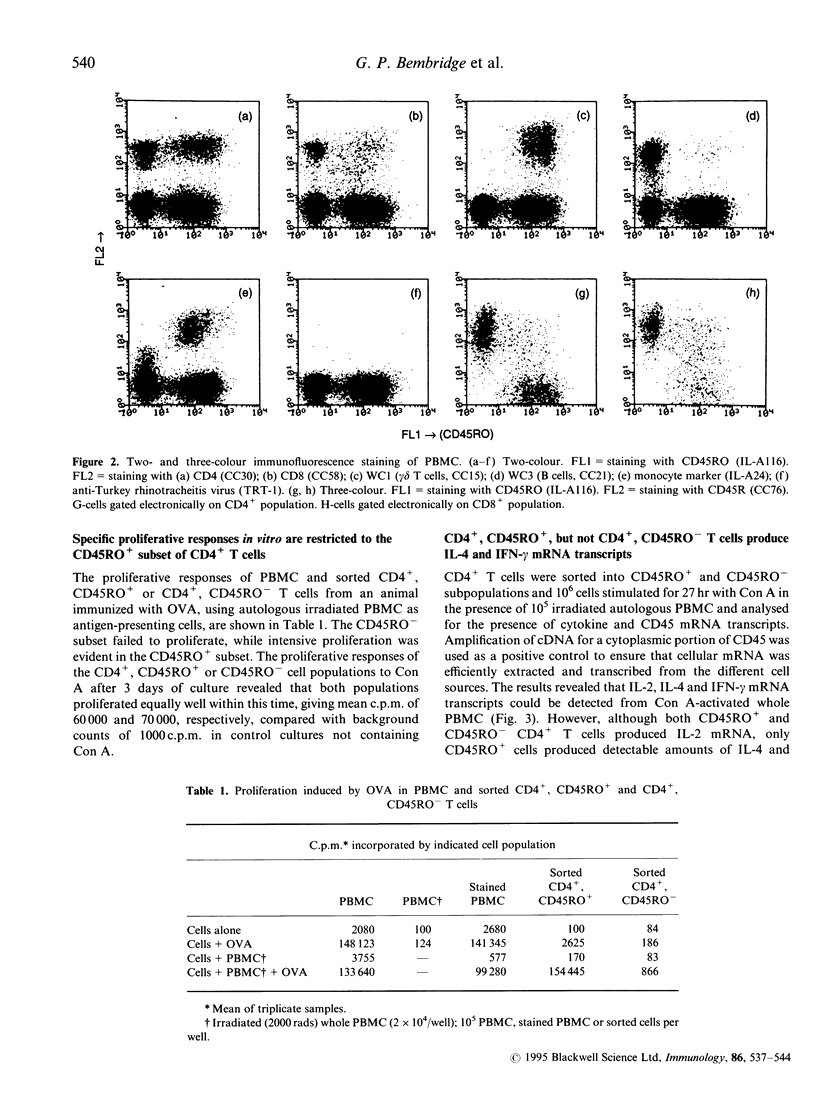
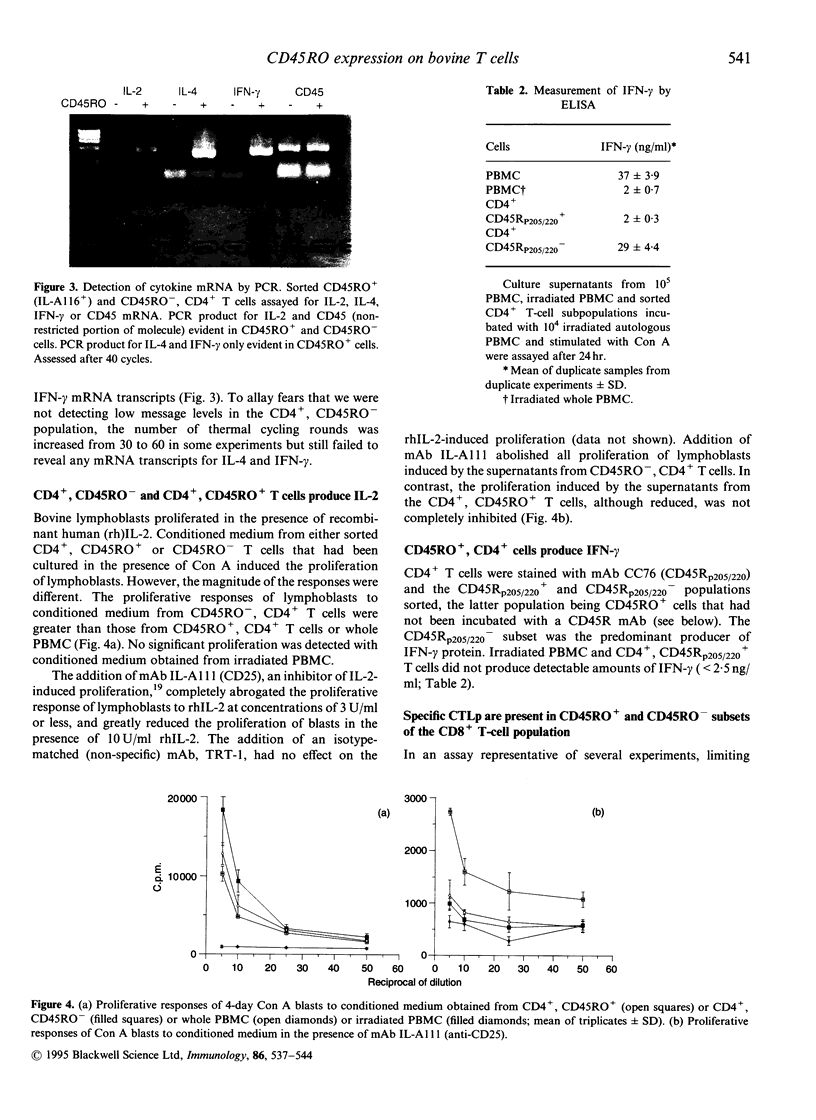
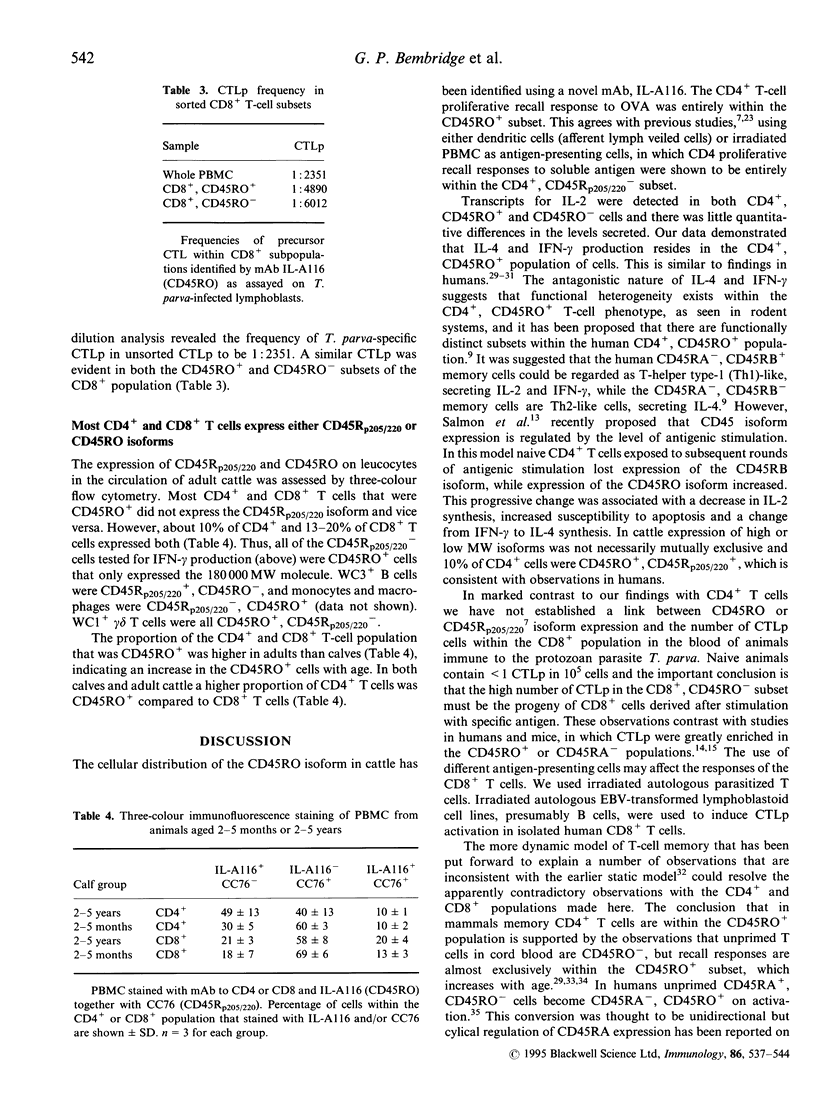
The 180,000 MW isoform of CD45 (CD45RO) has been identified in cattle with a novel monoclonal antibody (mAb) (IL-A116). This has allowed a more precise analysis of T-cell function in relation to CD45 isoform expression. Within the CD4+ and CD8+ T-cell populations, CD45RO+ and CD45RO- subsets were evident. Most CD4+ and CD8+ T cells that expressed the CD45RO isoform did not express the 220,000 and 205,000 MW isoforms recognized by mAb CC76. In contrast, the WC1+, CD2-, CD4-, CD8-, gamma delta T-cell receptor (TCR)+ T cells in bovine peripheral blood mononuclear cells (PBMC) were all CD45RO+. Monocytes and granulocytes were CD45RO+ but B cells were CD45RO-. Sorting experiments with CD4+ T cells from an immunized calf demonstrated that proliferative responses to ovalbumin (OVA) were entirely within the CD45RO+ subset. Following stimulation with concanavalin A (Con A) the CD45RO- subset of CD4+ T cells produced transcripts for interleukin-2 (IL-2) but not IL-4 or interferon-gamma (IFN-gamma), while the CD45RO+ subset produced mRNA for IL-2, IL-4 and IFN-gamma. Biologically active IL-2 was present in supernatants from both CD45RO+ and CD45RO-, CD4+ T cells, and IFN-gamma protein was identified by ELISA in supernatants from the CD45RO+ subset, confirming the production of cytokines implied by polymerase chain reaction (PCR). In contrast, sorting experiments with CD8+ T cells from animals immune to the protozoan parasite Theileria parva revealed substantial numbers of cytotoxic T-lymphocyte precursors in both the CD45RO+ and CD45RO- subsets. Thus it appears that although all antigenically primed CD4+ T cells remain CD45RO+, and expression of this molecule consequently identifies memory cells within PBMC, antigenically primed CD8+ T cells down-regulate CD45RO expression after activation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Borthwick N., Salmon M., Gombert W., Bofill M., Shamsadeen N., Pilling D., Pett S., Grundy J. E., Janossy G. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. J Exp Med. 1993 Aug 1;178(2):427–438. doi: 10.1084/jem.178.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Bell E. B., Sparshott S. M. Interconversion of CD45R subsets of CD4 T cells in vivo. Nature. 1990 Nov 8;348(6297):163–166. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- Beverley P. C. Is T-cell memory maintained by crossreactive stimulation? Immunol Today. 1990 Jun;11(6):203–205. doi: 10.1016/0167-5699(90)90083-l. [DOI] [PubMed] [Google Scholar]
- Clevers H., MacHugh N. D., Bensaid A., Dunlap S., Baldwin C. L., Kaushal A., Iams K., Howard C. J., Morrison W. I. Identification of a bovine surface antigen uniquely expressed on CD4-CD8- T cell receptor gamma/delta+ T lymphocytes. Eur J Immunol. 1990 Apr;20(4):809–817. doi: 10.1002/eji.1830200415. [DOI] [PubMed] [Google Scholar]
- Collins R. A., Tayton H. K., Gelder K. I., Britton P., Oldham G. Cloning and expression of bovine and porcine interleukin-2 in baculovirus and analysis of species cross-reactivity. Vet Immunol Immunopathol. 1994 Apr;40(4):313–324. doi: 10.1016/0165-2427(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Dohlsten M., Hedlund G., Sjögren H. O., Carlsson R. Two subsets of human CD4+ T helper cells differing in kinetics and capacities to produce interleukin 2 and interferon-gamma can be defined by the Leu-18 and UCHL1 monoclonal antibodies. Eur J Immunol. 1988 Aug;18(8):1173–1178. doi: 10.1002/eji.1830180805. [DOI] [PubMed] [Google Scholar]
- Ellis J. A., Baldwin C. L., MacHugh N. D., Bensaid A., Teale A. J., Goddeeris B. M., Morrison W. I. Characterization by a monoclonal antibody and functional analysis of a subset of bovine T lymphocytes that express BoT8, a molecule analogous to human CD8. Immunology. 1986 Jul;58(3):351–358. [PMC free article] [PubMed] [Google Scholar]
- Gray D., Matzinger P. T cell memory is short-lived in the absence of antigen. J Exp Med. 1991 Nov 1;174(5):969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannet I., Erkeller-Yuksel F., Lydyard P., Deneys V., DeBruyère M. Developmental and maturational changes in human blood lymphocyte subpopulations. Immunol Today. 1992 Jun;13(6):215–218. doi: 10.1016/0167-5699(92)90157-3. [DOI] [PubMed] [Google Scholar]
- Hovis R. R., Donovan J. A., Musci M. A., Motto D. G., Goldman F. D., Ross S. E., Koretzky G. A. Rescue of signaling by a chimeric protein containing the cytoplasmic domain of CD45. Science. 1993 Apr 23;260(5107):544–546. doi: 10.1126/science.8475387. [DOI] [PubMed] [Google Scholar]
- Howard C. J., Naessens J. Summary of workshop findings for cattle (tables 1 and 2). Vet Immunol Immunopathol. 1993 Nov;39(1-3):25–47. doi: 10.1016/0165-2427(93)90161-v. [DOI] [PubMed] [Google Scholar]
- Howard C. J., Parsons K. R., Jones B. V., Sopp P., Pocock D. H. Two monoclonal antibodies (CC17, CC29) recognizing an antigen (Bo5) on bovine T lymphocytes, analogous to human CD5. Vet Immunol Immunopathol. 1988 Sep;19(2):127–139. doi: 10.1016/0165-2427(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Howard C. J., Sopp P., Parsons K. R. L-selectin expression differentiates T cells isolated from different lymphoid tissues in cattle but does not correlate with memory. Immunology. 1992 Oct;77(2):228–234. [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Sopp P., Parsons K. R., McKeever D. J., Taracha E. L., Jones B. V., MacHugh N. D., Morrison W. I. Distinction of naive and memory BoCD4 lymphocytes in calves with a monoclonal antibody, CC76, to a restricted determinant of the bovine leukocyte-common antigen, CD45. Eur J Immunol. 1991 Sep;21(9):2219–2226. doi: 10.1002/eji.1830210933. [DOI] [PubMed] [Google Scholar]
- Johnson P., Greenbaum L., Bottomly K., Trowbridge I. S. Identification of the alternatively spliced exons of murine CD45 (T200) required for reactivity with B220 and other T200-restricted antibodies. J Exp Med. 1989 Mar 1;169(3):1179–1184. doi: 10.1084/jem.169.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. B., Prickett K. S., Larsen A., Grabstein K., Weaver M., Wilson C. B. Restricted production of interleukin 4 by activated human T cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9743–9747. doi: 10.1073/pnas.85.24.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay C. R., Marston W. L., Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990 Mar 1;171(3):801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D., Powrie F. Memory CD4+ T cells in man form two distinct subpopulations, defined by their expression of isoforms of the leucocyte common antigen, CD45. Immunology. 1990 Aug;70(4):427–433. [PMC free article] [PubMed] [Google Scholar]
- McKeever D. J., Taracha E. L., Innes E. L., MacHugh N. D., Awino E., Goddeeris B. M., Morrison W. I. Adoptive transfer of immunity to Theileria parva in the CD8+ fraction of responding efferent lymph. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1959–1963. doi: 10.1073/pnas.91.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie C. A., McLean A., Alcock C., Beverley P. C. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992 Nov 19;360(6401):264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- Naessens J., Sileghem M., MacHugh N., Park Y. H., Davis W. C., Toye P. Selection of BoCD25 monoclonal antibodies by screening mouse L cells transfected with the bovine p55-interleukin-2 (IL-2) receptor gene. Immunology. 1992 Jun;76(2):305–309. [PMC free article] [PubMed] [Google Scholar]
- Oehen S., Waldner H., Kündig T. M., Hengartner H., Zinkernagel R. M. Antivirally protective cytotoxic T cell memory to lymphocytic choriomeningitis virus is governed by persisting antigen. J Exp Med. 1992 Nov 1;176(5):1273–1281. doi: 10.1084/jem.176.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein D. M., Yamada A., Schlossman S. F., Morimoto C. Cyclic regulation of CD45 isoform expression in a long term human CD4+CD45RA+ T cell line. J Immunol. 1991 Feb 15;146(4):1175–1183. [PubMed] [Google Scholar]
- Salmon M., Pilling D., Borthwick N. J., Viner N., Janossy G., Bacon P. A., Akbar A. N. The progressive differentiation of primed T cells is associated with an increasing susceptibility to apoptosis. Eur J Immunol. 1994 Apr;24(4):892–899. doi: 10.1002/eji.1830240417. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Sparshott S. M., Bell E. B., Sarawar S. R. CD45R CD4 T cell subset-reconstituted nude rats: subset-dependent survival of recipients and bi-directional isoform switching. Eur J Immunol. 1991 Apr;21(4):993–1000. doi: 10.1002/eji.1830210420. [DOI] [PubMed] [Google Scholar]
- Streuli M., Hall L. R., Saga Y., Schlossman S. F., Saito H. Differential usage of three exons generates at least five different mRNAs encoding human leukocyte common antigens. J Exp Med. 1987 Nov 1;166(5):1548–1566. doi: 10.1084/jem.166.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Matsuyama T., Morimoto C., Schlossman S. F., Saito H. Identification of the sequence required for expression of the 2H4 epitope on the human leukocyte common antigens. J Exp Med. 1987 Nov 1;166(5):1567–1572. doi: 10.1084/jem.166.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taracha E. L., Goddeeris B. M., Scott J. R., Morrison W. I. Standardization of a technique for analysing the frequency of parasite-specific cytotoxic T lymphocyte precursors in cattle immunized with Theileria parva. Parasite Immunol. 1992 Mar;14(2):143–154. doi: 10.1111/j.1365-3024.1992.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Terry L. A., Brown M. H., Beverley P. C. The monoclonal antibody, UCHL1, recognizes a 180,000 MW component of the human leucocyte-common antigen, CD45. Immunology. 1988 Jun;64(2):331–336. [PMC free article] [PubMed] [Google Scholar]
- Tonks N. K., Charbonneau H., Diltz C. D., Fischer E. H., Walsh K. A. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry. 1988 Nov 29;27(24):8695–8701. doi: 10.1021/bi00424a001. [DOI] [PubMed] [Google Scholar]
- Williams D. J., Newson J., Naessens J. Quantitation of bovine immunoglobulin isotypes and allotypes using monoclonal antibodies. Vet Immunol Immunopathol. 1990 Mar;24(3):267–283. doi: 10.1016/0165-2427(90)90042-q. [DOI] [PubMed] [Google Scholar]