Abstract
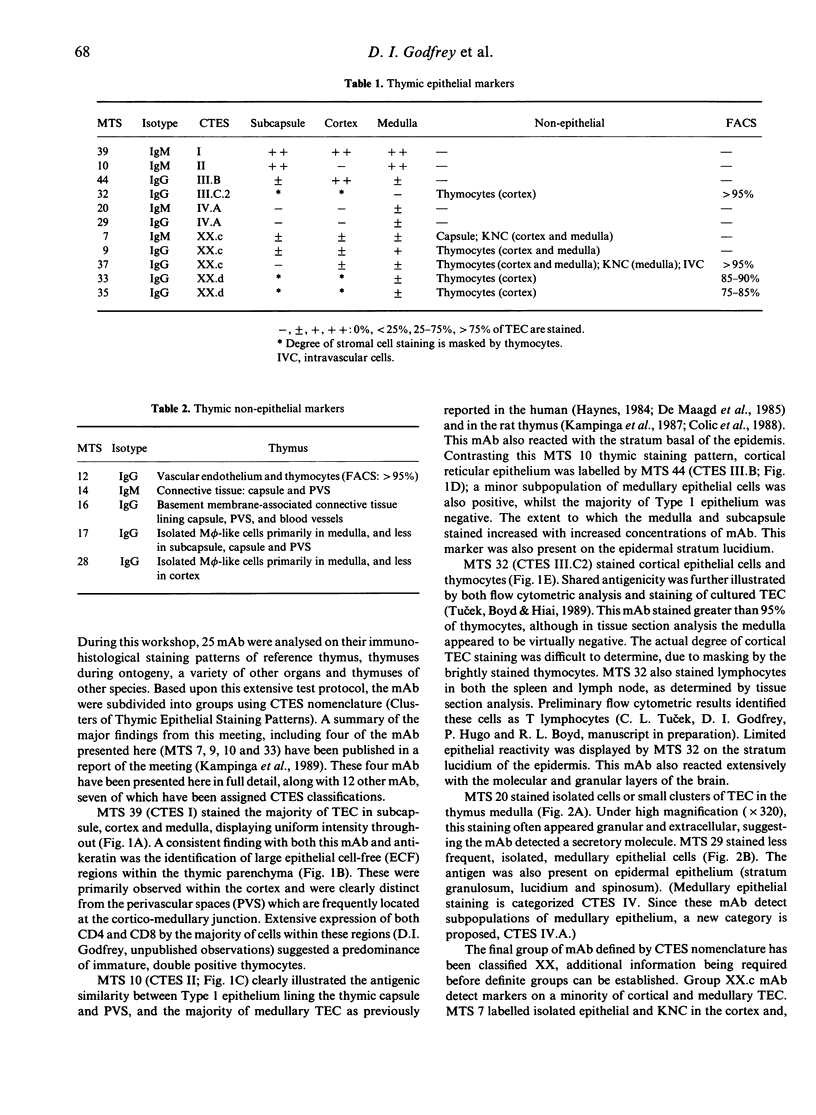
Sixteen monoclonal antibodies (mAb) were produced against mouse thymic stromal elements. These mAb fell into two groups of reactivity: (i) thymic epithelial markers (screened and presented according to the guidelines proposed in the 1989 Rolduc Thymic Epithelial Workshop); and (ii) non-epithelial thymic markers. Specificities of these mAb included extensive subpopulations of both epithelial and non-epithelial thymic stromal cells, as well as isolated stromal cells, demonstrating some of the complex microspecificities in existence within the thymic microenvironment. Furthermore, six of these mAb demonstrated shared antigenicity between thymocytes and thymic stromal cells, revealing greater similarities than previously recognized between these two components. Three mAb detected antigens illustrating three consecutive layers of the blood-thymus barrier: the vascular endothelium; connective tissue of the capsule and perivascular spaces; and the connective tissue associated with the basal laminae lining these regions. This study illustrates unequivocably that there are indeed complex and varied microenvironments existing within the thymus, and emphasizes the need for reclassification of these cells.
Full text
PDF

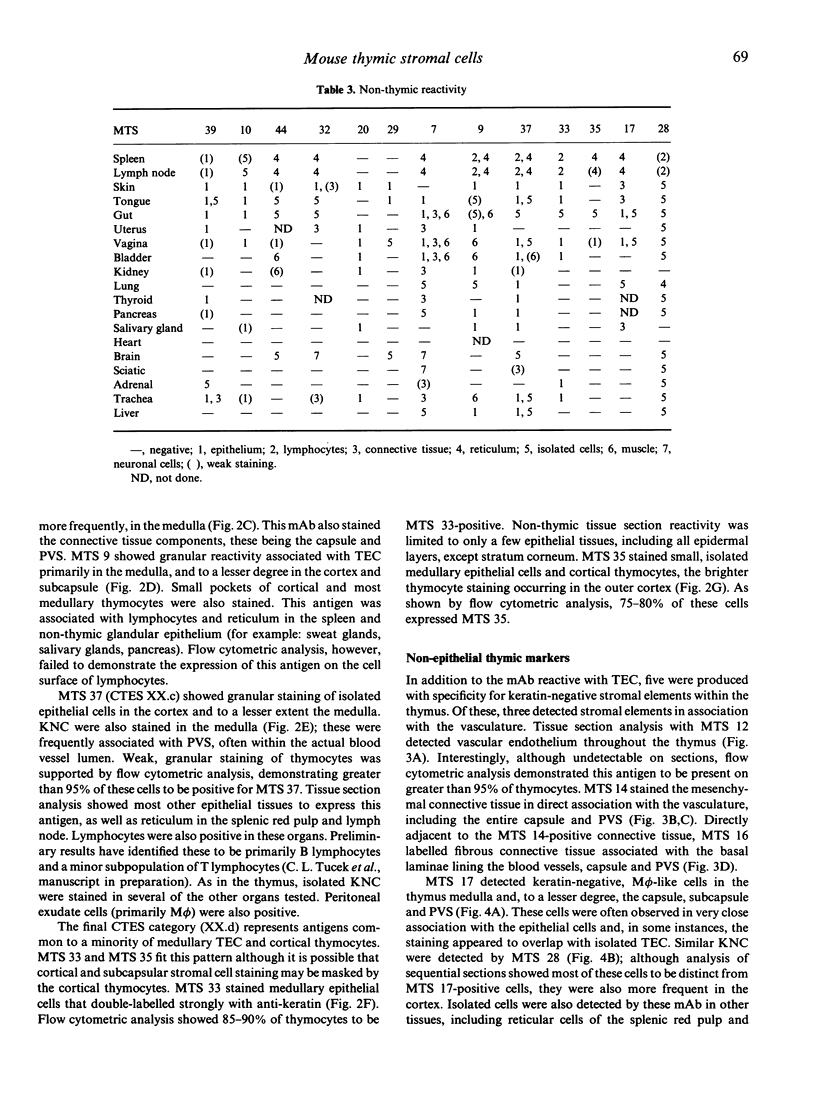
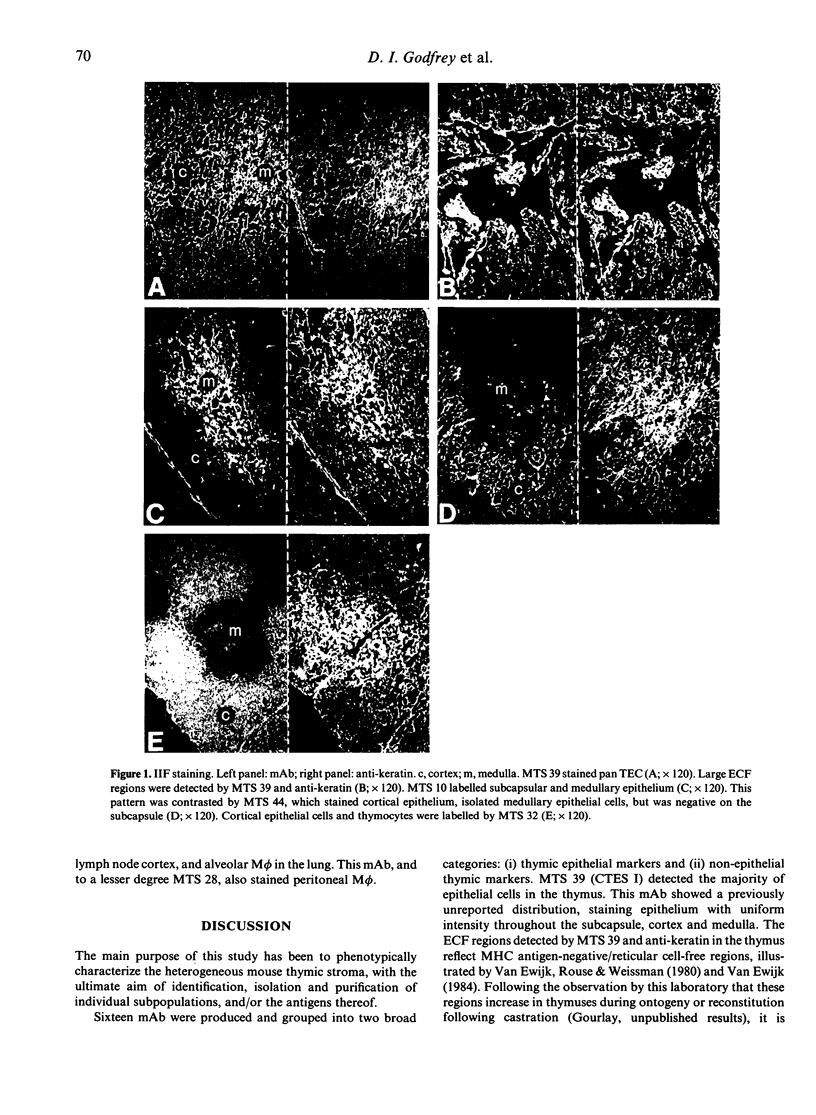
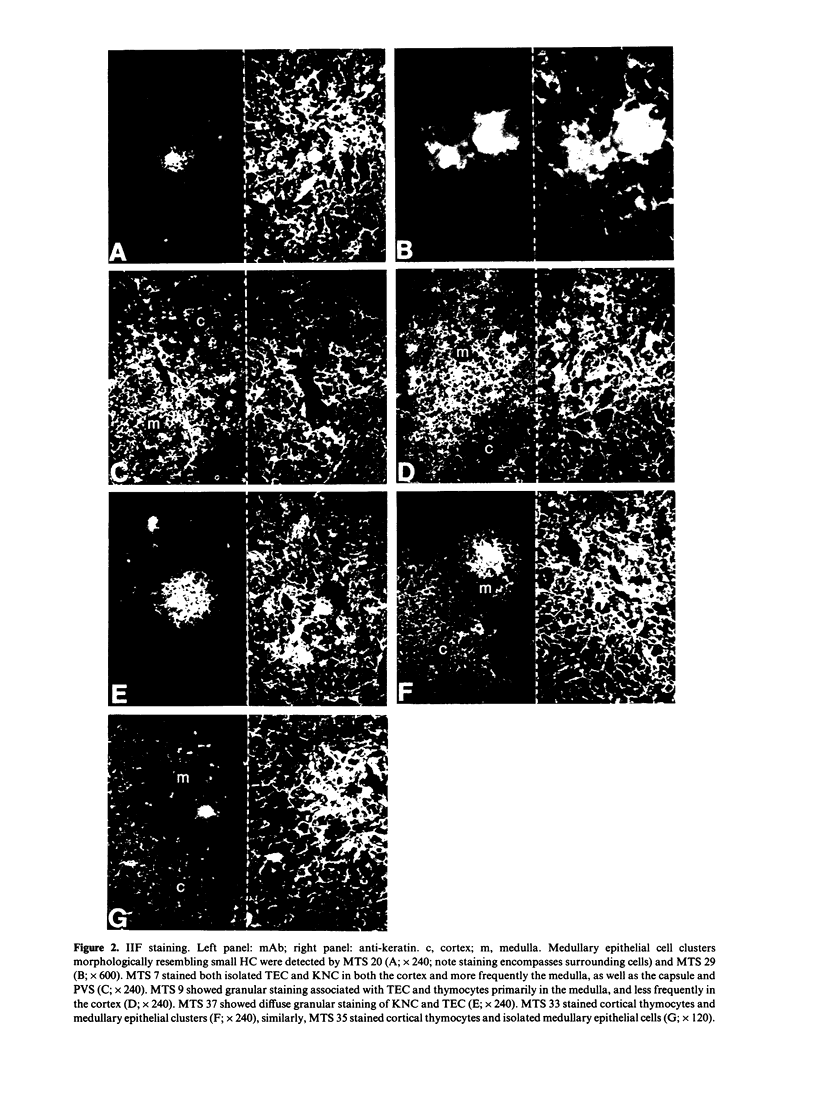
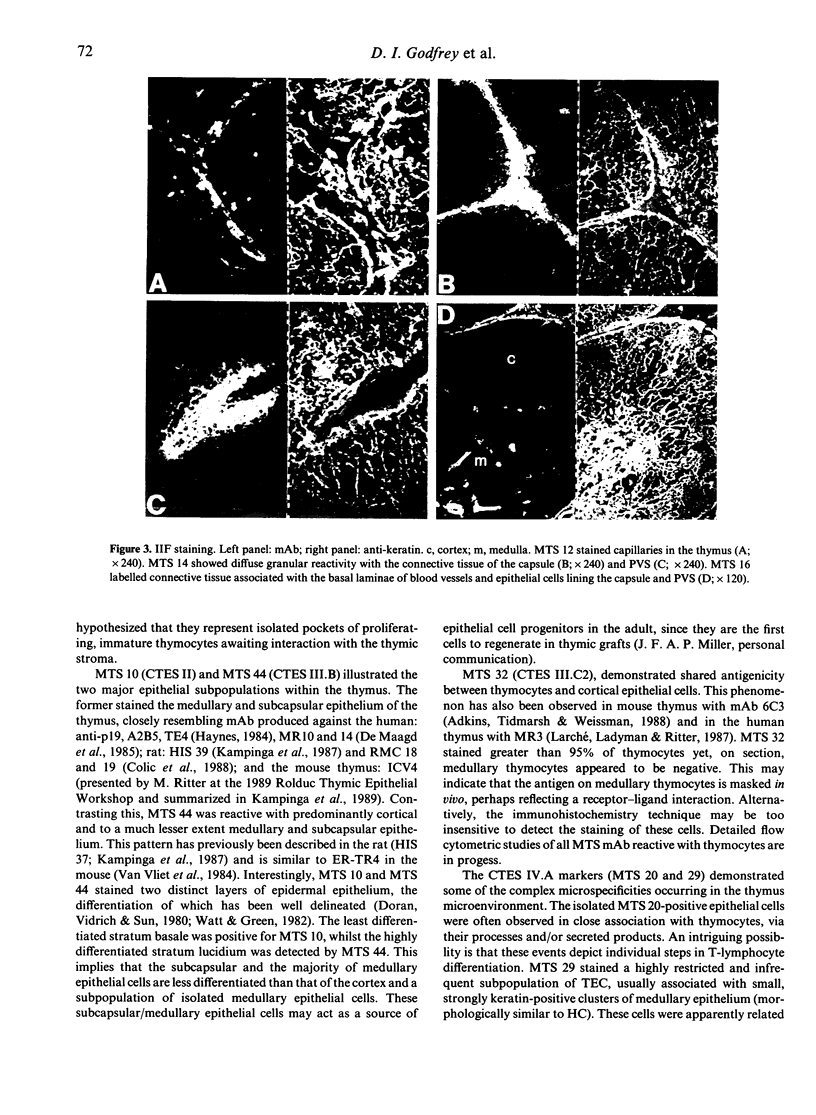


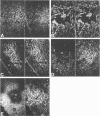
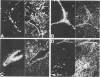
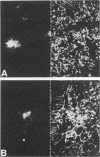
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Tidmarsh G. F., Weissman I. L. Normal thymic cortical epithelial cells developmentally regulate the expression of a B-lineage transformation-associated antigen. Immunogenetics. 1988;27(3):180–186. doi: 10.1007/BF00346584. [DOI] [PubMed] [Google Scholar]
- Berrih S., Savino W., Cohen S. Extracellular matrix of the human thymus: immunofluorescence studies on frozen sections and cultured epithelial cells. J Histochem Cytochem. 1985 Jul;33(7):655–664. doi: 10.1177/33.7.3891843. [DOI] [PubMed] [Google Scholar]
- Colić M., Matanović D., Hegedis L., Dujić A. Immunohistochemical characterization of rat thymic non-lymphoid cells. I. Epithelial and mesenchymal components defined by monoclonal antibodies. Immunology. 1988 Oct;65(2):277–284. [PMC free article] [PubMed] [Google Scholar]
- Denning S. M., Kurtzberg J., Le P. T., Tuck D. T., Singer K. H., Haynes B. F. Human thymic epithelial cells directly induce activation of autologous immature thymocytes. Proc Natl Acad Sci U S A. 1988 May;85(9):3125–3129. doi: 10.1073/pnas.85.9.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Haynes B. F. The human thymic microenvironment. Adv Immunol. 1984;36:87–142. doi: 10.1016/s0065-2776(08)60900-1. [DOI] [PubMed] [Google Scholar]
- Kampinga J., Berges S., Boyd R. L., Brekelmans P., Colić M., van Ewijk W., Kendall M. D., Ladyman H., Nieuwenhuis P., Ritter M. A. Thymic epithelial antibodies: immunohistological analysis and introduction of nomenclature. Thymus. 1989;13(3-4):165–173. [PubMed] [Google Scholar]
- Larche M., Lamb J. R., O'Hehir R. E., Imami-Shita N., Zanders E. D., Quint D. E., Moqbel R., Ritter M. A. Functional evidence for a monoclonal antibody that binds to the human IL-4 receptor. Immunology. 1988 Dec;65(4):617–622. [PMC free article] [PubMed] [Google Scholar]
- Lobach D. F., Scearce R. M., Haynes B. F. The human thymic microenvironment. Phenotypic characterization of Hassall's bodies with the use of monoclonal antibodies. J Immunol. 1985 Jan;134(1):250–257. [PubMed] [Google Scholar]
- Stutman O. Intrathymic and extrathymic T cell maturation. Immunol Rev. 1978;42:138–184. doi: 10.1111/j.1600-065x.1978.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Van Ewijk W. Immunohistology of lymphoid and non-lymphoid cells in the thymus in relation to T lymphocyte differentiation. Am J Anat. 1984 Jul;170(3):311–330. doi: 10.1002/aja.1001700307. [DOI] [PubMed] [Google Scholar]
- Van Ewijk W., Rouse R. V., Weissman I. L. Distribution of H-2 microenvironments in the mouse thymus. Immunoelectron microscopic identification of I-A and H-2K bearing cells. J Histochem Cytochem. 1980 Oct;28(10):1089–1099. doi: 10.1177/28.10.6999083. [DOI] [PubMed] [Google Scholar]
- Van Vliet E., Melis M., Van Ewijk W. Monoclonal antibodies to stromal cell types of the mouse thymus. Eur J Immunol. 1984 Jun;14(6):524–529. doi: 10.1002/eji.1830140608. [DOI] [PubMed] [Google Scholar]
- Watt F. M., Green H. Stratification and terminal differentiation of cultured epidermal cells. Nature. 1982 Feb 4;295(5848):434–436. doi: 10.1038/295434a0. [DOI] [PubMed] [Google Scholar]
- de Maagd R. A., MacKenzie W. A., Schuurman H. J., Ritter M. A., Price K. M., Broekhuizen R., Kater L. The human thymus microenvironment: heterogeneity detected by monoclonal anti-epithelial cell antibodies. Immunology. 1985 Apr;54(4):745–754. [PMC free article] [PubMed] [Google Scholar]
- de la Hera A., Marston W., Aranda C., Toribio M. L., Martinez C. Thymic stroma is required for the development of human T cell lineages in vitro. Int Immunol. 1989;1(5):471–478. doi: 10.1093/intimm/1.5.471. [DOI] [PubMed] [Google Scholar]
- van de Wijngaert F. P., Kendall M. D., Schuurman H. J., Rademakers L. H., Kater L. Heterogeneity of epithelial cells in the human thymus. An ultrastructural study. Cell Tissue Res. 1984;237(2):227–237. doi: 10.1007/BF00217140. [DOI] [PubMed] [Google Scholar]