Abstract
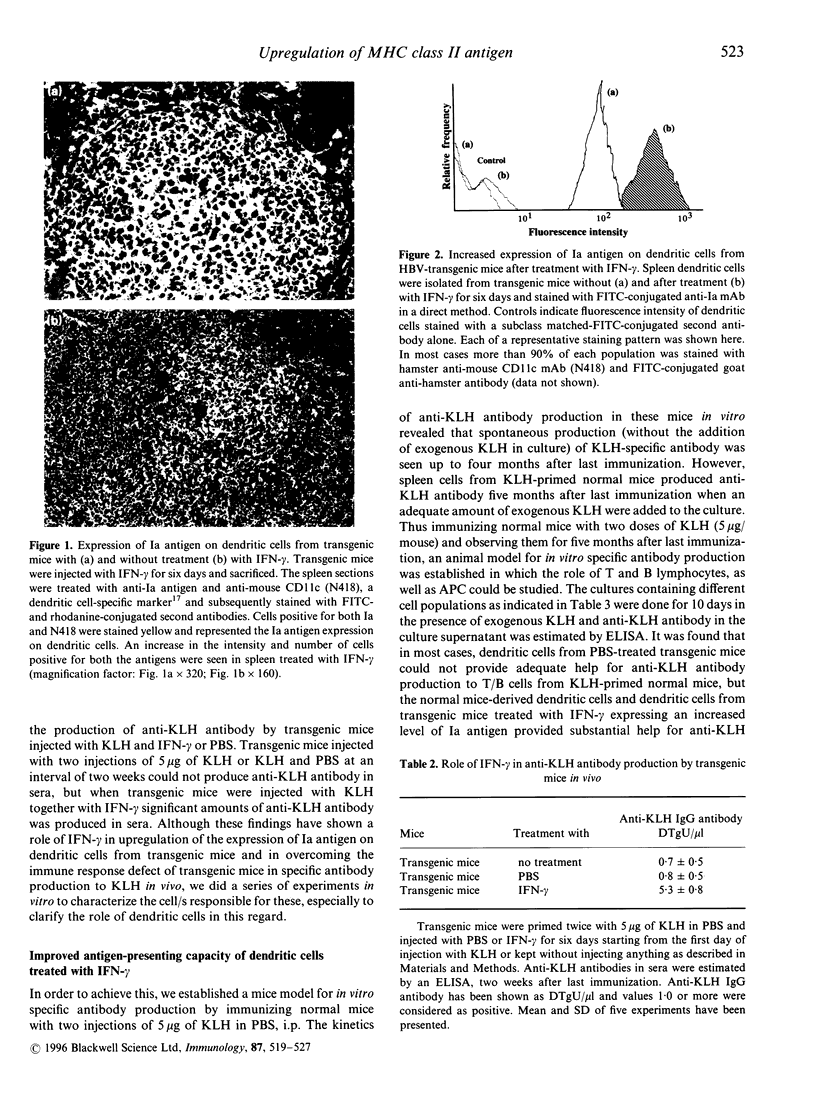
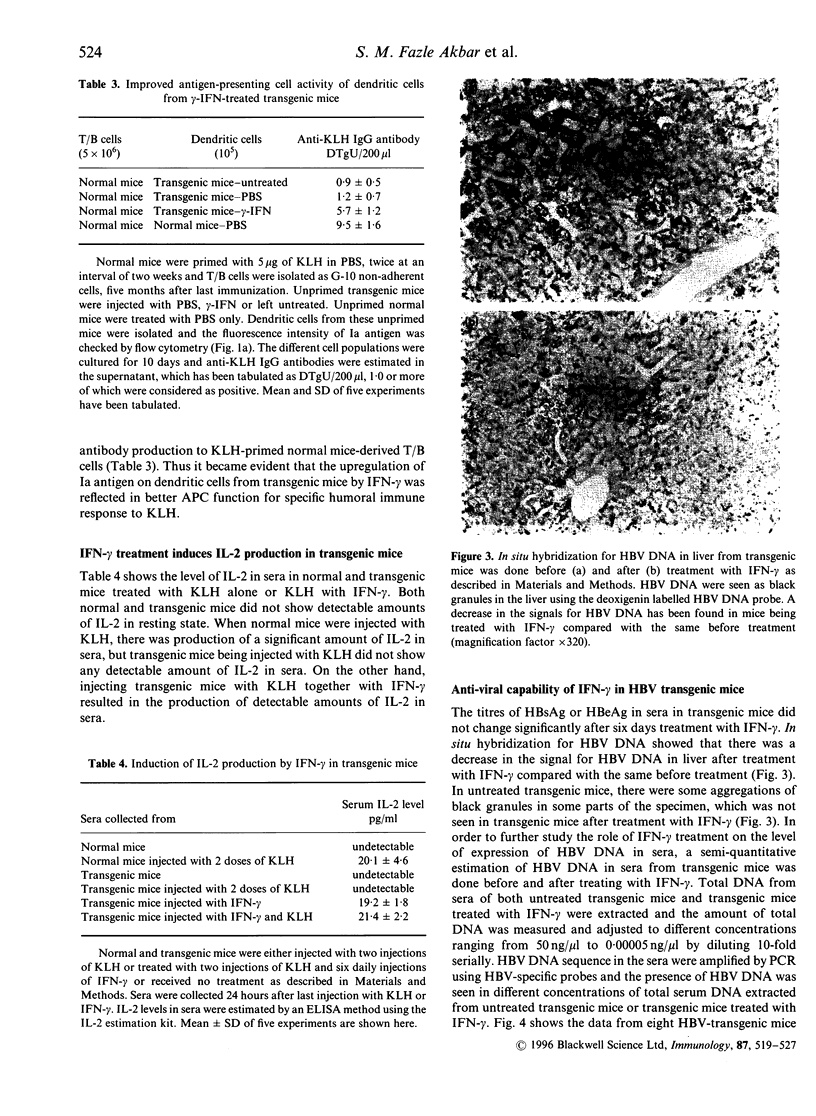
The experiments presented here were performed to see whether the level of expression of major histocompatibility complex (MHC) class II antigen (Ia antigen) on dendritic cells, one of the most critical antigen presenting cells (APC), influences the humoral immune response in hepatitis B virus (HBV) transgenic mice. We have reported that transgenic mice had a low responsiveness in specific antibody production to keyhole limpet haemocyanin (KLH), a T-cell dependent, HBV-unrelated antigen compared with the age, sex, and major histocompatibility-matched normal mice, due to a significantly lower T-cell stimulatory capacity of transgenic mice-derived dendritic cells, possibly as a result of significantly lower level of Ia antigen. Immunohistochemical staining has shown that treatment of transgenic mice with mouse recombinant interferon-gamma (IFN-gamma), daily for six consecutive days resulted in an increased expression of Ia antigen on splenic dendritic cells. Again, flow cytometric analyses have further confirmed the significant increase in the expression of Ia antigen on dendritic cells, isolated from transgenic mice treated with IFN-gamma compared with the same from the untreated or phosphate-buffered saline (PBS)-treated transgenic mice. Transgenic mice immunized with two optimum doses of KLH (5 micrograms/mouse) could not produce anti-KLH antibodies in sera, but injecting transgenic mice with the same doses of KLH together with IFN-gamma resulted in the production of anti-KLH antibodies in sera. Again, KLH-primed normal mice-derived T/B lymphocytes produced anti-KLH antibody, when cultured with dendritic cells from IFN-gamma-treated transgenic mice expressing a higher level of Ia antigen, but not with the same from PBS-treated or untreated transgenic mice. Treatment of transgenic mice with IFN-gamma resulted in a reduced level of hepatitis B virus (HBV) DNA in liver and in sera. These experiments have shown that the level of expression of Ia antigen on dendritic cells is a critical factor for its APC capability and its modulation of IFN-gamma may be used for immune therapy in HBV carriers.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar S. M., Onji M., Inaba K., Yamamura K., Ohta Y. Low responsiveness of hepatitis B virus-transgenic mice in antibody response to T-cell-dependent antigen: defect in antigen-presenting activity of dendritic cells. Immunology. 1993 Mar;78(3):468–475. [PMC free article] [PubMed] [Google Scholar]
- Araki K., Miyazaki J., Hino O., Tomita N., Chisaka O., Matsubara K., Yamamura K. Expression and replication of hepatitis B virus genome in transgenic mice. Proc Natl Acad Sci U S A. 1989 Jan;86(1):207–211. doi: 10.1073/pnas.86.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basham T. Y., Merigan T. C. Recombinant interferon-gamma increases HLA-DR synthesis and expression. J Immunol. 1983 Apr;130(4):1492–1494. [PubMed] [Google Scholar]
- Crowley M., Inaba K., Witmer-Pack M., Steinman R. M. The cell surface of mouse dendritic cells: FACS analyses of dendritic cells from different tissues including thymus. Cell Immunol. 1989 Jan;118(1):108–125. doi: 10.1016/0008-8749(89)90361-4. [DOI] [PubMed] [Google Scholar]
- Di Bisceglie A. M., Rustgi V. K., Kassianides C., Lisker-Melman M., Park Y., Waggoner J. G., Hoofnagle J. H. Therapy of chronic hepatitis B with recombinant human alpha and gamma interferon. Hepatology. 1990 Feb;11(2):266–270. doi: 10.1002/hep.1840110217. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Mier J. W. Lymphokines. N Engl J Med. 1987 Oct 8;317(15):940–945. doi: 10.1056/NEJM198710083171506. [DOI] [PubMed] [Google Scholar]
- Dusheiko G. M., Hoofnagle J. H., Cooksley W. G., James S. P., Jones E. A. Synthesis of antibodies to hepatitis B virus by cultured lymphocytes from chronic hepatitis B surface antigen carriers. J Clin Invest. 1983 May;71(5):1104–1113. doi: 10.1172/JCI110860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea E., Iglesias A., Salazar M., Morimoto C., Kruskall M. S., Awdeh Z., Schlossman S. F., Alper C. A., Yunis E. J. The cellular basis for lack of antibody response to hepatitis B vaccine in humans. J Exp Med. 1991 Mar 1;173(3):531–538. doi: 10.1084/jem.173.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernan A., Cayzer C. J., Cooksley W. G. HBsAg-induced antigen-specific T and B lymphocyte responses in chronic hepatitis B virus carriers and immune individuals. Clin Exp Immunol. 1989 May;76(2):222–226. [PMC free article] [PubMed] [Google Scholar]
- Guidotti L. G., Ando K., Hobbs M. V., Ishikawa T., Runkel L., Schreiber R. D., Chisari F. V. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3764–3768. doi: 10.1073/pnas.91.9.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengel H., Lucin P., Jonjić S., Ruppert T., Koszinowski U. H. Restoration of cytomegalovirus antigen presentation by gamma interferon combats viral escape. J Virol. 1994 Jan;68(1):289–297. doi: 10.1128/jvi.68.1.289-297.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Resting and sensitized T lymphocytes exhibit distinct stimulatory (antigen-presenting cell) requirements for growth and lymphokine release. J Exp Med. 1984 Dec 1;160(6):1717–1735. doi: 10.1084/jem.160.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. C., Volkman D. J., Whalen G., Fauci A. S. In vitro antigen-induced, antigen-specific antibody production in man. Specific and polyclonal components, kinetics, and cellular requirements. J Exp Med. 1981 Oct 1;154(4):1043–1057. doi: 10.1084/jem.154.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J. Y., Bain V. G., Naoumov N. V., Smith H. M., Alexander G. J., Williams R. Effect of interferon-gamma on hepatitis B viral antigen expression in primary hepatocyte culture. Hepatology. 1991 Dec;14(6):975–979. [PubMed] [Google Scholar]
- Le Gros G., Ben-Sasson S. Z., Seder R., Finkelman F. D., Paul W. E. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990 Sep 1;172(3):921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T., McLachlan A., Iino S., Koike K., Kurokawa K., Milich D. R. The serology of chronic hepatitis B infection revisited. J Clin Invest. 1993 Jun;91(6):2586–2595. doi: 10.1172/JCI116497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T., Thornton G. B., Iino S., Kurokawa K., Milich D. R. Use of anti-peptide antibodies for the design of antigen-specific immune complex assays. J Immunol Methods. 1992 Oct 19;155(1):65–75. doi: 10.1016/0022-1759(92)90272-u. [DOI] [PubMed] [Google Scholar]
- Metlay J. P., Witmer-Pack M. D., Agger R., Crowley M. T., Lawless D., Steinman R. M. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990 May 1;171(5):1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momburg F., Koch N., Möller P., Moldenhauer G., Butcher G. W., Hämmerling G. J. Differential expression of Ia and Ia-associated invariant chain in mouse tissues after in vivo treatment with IFN-gamma. J Immunol. 1986 Feb 1;136(3):940–948. [PubMed] [Google Scholar]
- Nowicki M. J., Tong M. J., Nair P. V., Stevenson D. Impaired antibody synthesis in patients with chronic hepatitis B infection. Hepatology. 1986 Mar-Apr;6(2):180–185. doi: 10.1002/hep.1840060205. [DOI] [PubMed] [Google Scholar]
- Peeters C. C., Tenbergen-Meekes A. M., Heijnen C. J., Poolman J. T., Zegers J. M., Rijkers G. T. Interferon-gamma and interleukin-6 augment the human in vitro antibody response to the Haemophilus influenzae type b polysaccharide. J Infect Dis. 1992 Jun;165 (Suppl 1):S161–S162. doi: 10.1093/infdis/165-supplement_1-s161. [DOI] [PubMed] [Google Scholar]
- Peters M., Vierling J., Gershwin M. E., Milich D., Chisari F. V., Hoofnagle J. H. Immunology and the liver. Hepatology. 1991 May;13(5):977–994. [PubMed] [Google Scholar]
- Ronchese F., Hausmann B., Le Gros G. Interferon-gamma- and interleukin-4-producing T cells can be primed on dendritic cells in vivo and do not require the presence of B cells. Eur J Immunol. 1994 May;24(5):1148–1154. doi: 10.1002/eji.1830240521. [DOI] [PubMed] [Google Scholar]
- Takashima H., Araki K., Miyazaki J., Yamamura K., Kimoto M. Characterization of T-cell tolerance to hepatitis B virus (HBV) antigen in transgenic mice. Immunology. 1992 Mar;75(3):398–405. [PMC free article] [PubMed] [Google Scholar]
- Tillmann H., Trautwein C., Walker D., Michitaka K., Kubicka S., Böker K., Manns M. Clinical relevance of mutations in the precore genome of the hepatitis B virus. Gut. 1995 Oct;37(4):568–573. doi: 10.1136/gut.37.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virelizier J. L., Perez N., Arenzana-Seisdedos F., Devos R. Pure interferon gamma enhances class II HLA antigens on human monocyte cell lines. Eur J Immunol. 1984 Jan;14(1):106–108. doi: 10.1002/eji.1830140120. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Y., Michael J. G. Orally inducible immune unresponsiveness is abrogated by IFN-gamma treatment. J Immunol. 1990 Jun 1;144(11):4163–4165. [PubMed] [Google Scholar]