Abstract
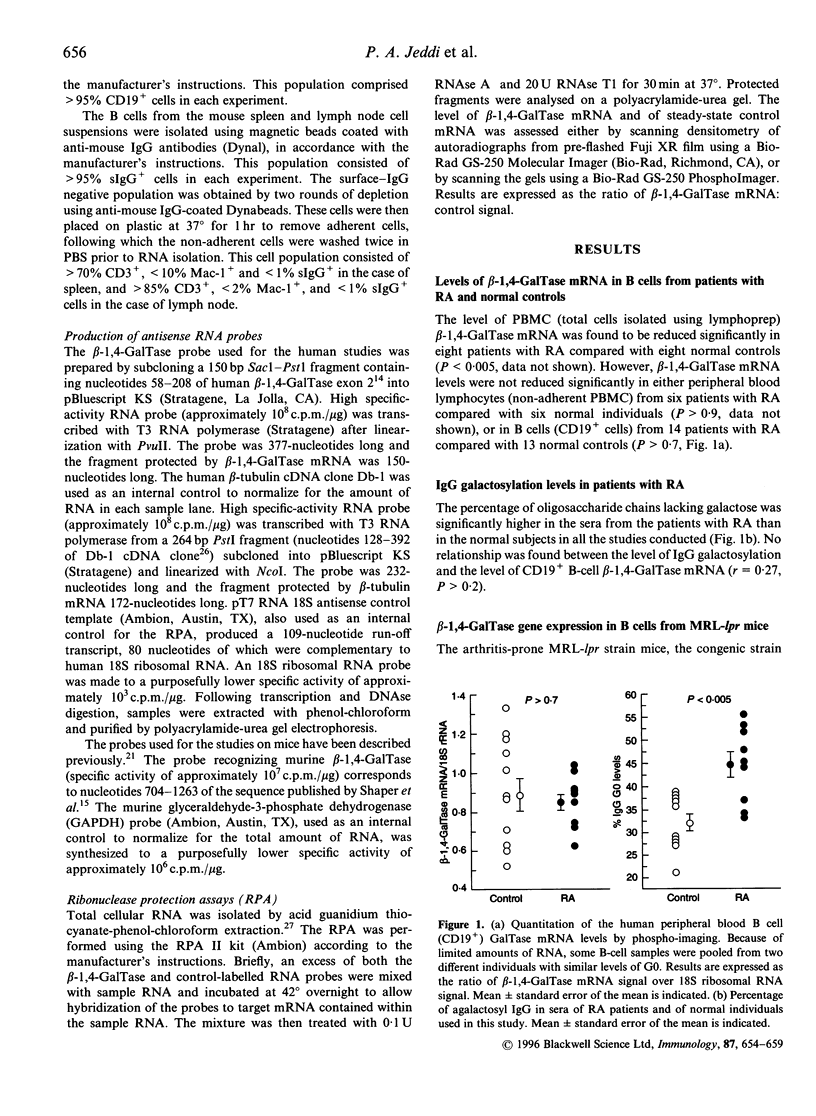
Reduced galactosylation of immunoglobulin G (IgG) is well documented in rheumatoid arthritis (RA), but the reason for this defect is still unknown. There is some evidence supporting a defect in the biosynthetic pathway, and a reduction in the level of beta-1,4-galactosyltransferase (beta-1,4-GalTase) enzyme activity. Since glycosyltransferases are, in general, regulated at the level of transcription, we have measured the level of beta-1,4-GalTase gene expression in B cells from patients with RA and normal control individuals. We found no significant difference in mRNA levels for the transferase in these two groups (P > 0.7). MRL/Mp-lpr/lpr (MRL-lpr) mice develop a spontaneous arthritis with increased levels of agalactosyl IgG (G0). In spite of a significant reduction in the level of beta-1,4-GalTase mRNA in total spleen lymphocytes from MRL-lpr mice compared with the congenic MRL/Mp-(+/+) (MRL-(+/+) mice and with CBA/Ca mice, we found comparable levels of the beta-1,4-GalTase mRNA in purified B cells from both spleen and lymph nodes of the three strains. Amongst the lymphoid compartments examined, the spleen and peripheral blood were found to be the major contributors of G0 in MRL-lpr mice. These data indicate that in neither human RA, nor in an animal model of this disease, is reduced IgG galactosylation caused by impaired expression of the beta-1,4-GalTase gene in B lymphocytes. Furthermore, splenic B cells, which have normal levels of beta-1,4-GalTase mRNA, appear to be a major source of G0 in MRL-lpr mice.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Axford J. S., Alavi A., Bond A., Hay F. C. Differential B lymphocyte galactosyltransferase activity in the MRL mouse model of rheumatoid arthritis. Autoimmunity. 1994;17(2):157–163. doi: 10.3109/08916939409014671. [DOI] [PubMed] [Google Scholar]
- Axford J. S., Mackenzie L., Lydyard P. M., Hay F. C., Isenberg D. A., Roitt I. M. Reduced B-cell galactosyltransferase activity in rheumatoid arthritis. Lancet. 1987 Dec 26;2(8574):1486–1488. doi: 10.1016/s0140-6736(87)92621-3. [DOI] [PubMed] [Google Scholar]
- Axford J. S., Sumar N., Alavi A., Isenberg D. A., Young A., Bodman K. B., Roitt I. M. Changes in normal glycosylation mechanisms in autoimmune rheumatic disease. J Clin Invest. 1992 Mar;89(3):1021–1031. doi: 10.1172/JCI115643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodman K. B., Hutchings P. R., Jeddi P. A., Delves P. J., Rook G. A., Sumar N., Roitt I. M., Lydyard P. M. IgG glycosylation in autoimmune-prone strains of mice. Clin Exp Immunol. 1994 Jan;95(1):103–107. doi: 10.1111/j.1365-2249.1994.tb06022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodman K. B., Sumar N., Mackenzie L. E., Isenberg D. A., Hay F. C., Roitt I. M., Lydyard P. M. Lymphocytes from patients with rheumatoid arthritis produce agalactosylated IgG in vitro. Clin Exp Immunol. 1992 Jun;88(3):420–423. doi: 10.1111/j.1365-2249.1992.tb06465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A., Cooke A., Hay F. C. Glycosylation of IgG, immune complexes and IgG subclasses in the MRL-lpr/lpr mouse model of rheumatoid arthritis. Eur J Immunol. 1990 Oct;20(10):2229–2233. doi: 10.1002/eji.1830201011. [DOI] [PubMed] [Google Scholar]
- Bunnell B. A., Adams D. E., Kidd V. J. Transient expression of a p58 protein kinase cDNA enhances mammalian glycosyltransferase activity. Biochem Biophys Res Commun. 1990 Aug 31;171(1):196–203. doi: 10.1016/0006-291x(90)91376-4. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dwek R. A. Glycobiology: "towards understanding the function of sugars". Biochem Soc Trans. 1995 Feb;23(1):1–25. doi: 10.1042/bst0230001. [DOI] [PubMed] [Google Scholar]
- Field M. C., Amatayakul-Chantler S., Rademacher T. W., Rudd P. M., Dwek R. A. Structural analysis of the N-glycans from human immunoglobulin A1: comparison of normal human serum immunoglobulin A1 with that isolated from patients with rheumatoid arthritis. Biochem J. 1994 Apr 1;299(Pt 1):261–275. doi: 10.1042/bj2990261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Kobata A. IgG galactosylation--its biological significance and pathology. Mol Immunol. 1991 Dec;28(12):1333–1340. doi: 10.1016/0161-5890(91)90035-i. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Matsuta K., Takeuchi F., Kosuge E., Miyamoto T., Kobata A. Kinetic study of a galactosyltransferase in the B cells of patients with rheumatoid arthritis. Int Immunol. 1990;2(1):105–112. doi: 10.1093/intimm/2.1.105. [DOI] [PubMed] [Google Scholar]
- Hollis G. F., Douglas J. G., Shaper N. L., Shaper J. H., Stafford-Hollis J. M., Evans R. J., Kirsch I. R. Genomic structure of murine beta-1,4-galactosyltransferase. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1069–1075. doi: 10.1016/0006-291x(89)90782-1. [DOI] [PubMed] [Google Scholar]
- Imai Y., Yamashita Y., Osawa T. Enhancement of the activities of glycosyltransferases involved in the biosynthesis of mucin-type sugar chains in autoimmune MRL lpr/lpr mouse T cells. Mol Immunol. 1988 May;25(5):419–428. doi: 10.1016/0161-5890(88)90161-7. [DOI] [PubMed] [Google Scholar]
- Ishii N., Watanabe K. Aberrant expression of GM1 on lymph node cells of MRL/Mp-lpr/lpr mice: influences on the autoreactivities of anti-asialo GM1 antibodies. Autoimmunity. 1992;13(2):107–116. doi: 10.3109/08916939209001911. [DOI] [PubMed] [Google Scholar]
- Jeddi P. A., Lund T., Bodman K. B., Sumar N., Lydyard P. M., Pouncey L., Heath L. S., Kidd V. J., Delves P. J. Reduced galactosyltransferase mRNA levels are associated with the agalactosyl IgG found in arthritis-prone MRL-lpr/lpr strain mice. Immunology. 1994 Nov;83(3):484–488. [PMC free article] [PubMed] [Google Scholar]
- Katagiri T., Mori T., Nakano T., Ueno K., Kano K. Aberrant expression of Forssman and Paul-Bunnell antigens on lymph node cells of MRL/Mp-lpr/lpr mice. J Immunol. 1984 Dec;133(6):3143–3148. [PubMed] [Google Scholar]
- Keusch J., Lydyard P. M., Isenberg D. A., Delves P. J. beta 1,4-Galactosyltransferase activity in B cells detected using a simple ELISA-based assay. Glycobiology. 1995 Jun;5(4):365–700. doi: 10.1093/glycob/5.4.365. [DOI] [PubMed] [Google Scholar]
- Kumpel B. M., Rademacher T. W., Rook G. A., Williams P. J., Wilson I. B. Galactosylation of human IgG monoclonal anti-D produced by EBV-transformed B-lymphoblastoid cell lines is dependent on culture method and affects Fc receptor-mediated functional activity. Hum Antibodies Hybridomas. 1994;5(3-4):143–151. [PubMed] [Google Scholar]
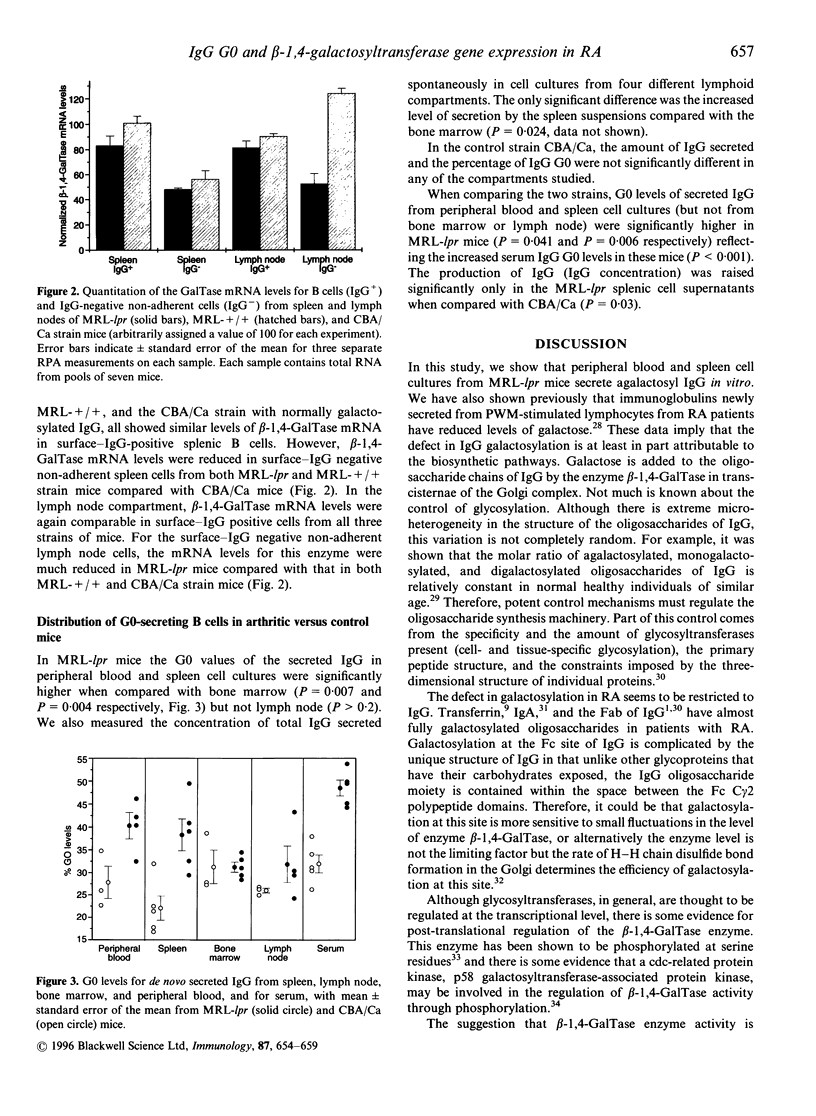
- Malhotra R., Wormald M. R., Rudd P. M., Fischer P. B., Dwek R. A., Sim R. B. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med. 1995 Mar;1(3):237–243. doi: 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- Mengle-Gaw L., McCoy-Haman M. F., Tiemeier D. C. Genomic structure and expression of human beta-1,4-galactosyltransferase. Biochem Biophys Res Commun. 1991 May 15;176(3):1269–1276. doi: 10.1016/0006-291x(91)90423-5. [DOI] [PubMed] [Google Scholar]
- Mullis P. E., Lund T., Patel M. S., Brook C. G., Brickell P. M. Regulation of human growth hormone receptor gene expression by human growth hormone in a human hepatoma cell line. Mol Cell Endocrinol. 1991 Apr;76(1-3):125–133. doi: 10.1016/0303-7207(91)90267-v. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985 Aug 1;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Roitt I. M., Isenberg D. A., Dwek R. A., Ansell B. M., Rademacher T. W. Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet. 1988 Apr 30;1(8592):966–969. doi: 10.1016/s0140-6736(88)91781-3. [DOI] [PubMed] [Google Scholar]
- Rademacher T. W., Williams P., Dwek R. A. Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6123–6127. doi: 10.1073/pnas.91.13.6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R. N., Shaper N. L., Shaper J. H. Bovine beta 1----4-galactosyltransferase: two sets of mRNA transcripts encode two forms of the protein with different amino-terminal domains. In vitro translation experiments demonstrate that both the short and the long forms of the enzyme are type II membrane-bound glycoproteins. J Biol Chem. 1990 Feb 25;265(6):3324–3331. [PubMed] [Google Scholar]
- Shaper N. L., Hollis G. F., Douglas J. G., Kirsch I. R., Shaper J. H. Characterization of the full length cDNA for murine beta-1,4-galactosyltransferase. Novel features at the 5'-end predict two translational start sites at two in-frame AUGs. J Biol Chem. 1988 Jul 25;263(21):10420–10428. [PubMed] [Google Scholar]
- Shaper N. L., Shaper J. H., Bertness V., Chang H., Kirsch I. R., Hollis G. F. The human galactosyltransferase gene is on chromosome 9 at band p13. Somat Cell Mol Genet. 1986 Nov;12(6):633–636. doi: 10.1007/BF01671948. [DOI] [PubMed] [Google Scholar]
- Shields J. G., Turner M. W. The importance of antibody quality in sandwich ELISA systems. Evaluation of selected commercial reagents. J Immunol Methods. 1986 Feb 27;87(1):29–33. doi: 10.1016/0022-1759(86)90340-6. [DOI] [PubMed] [Google Scholar]
- Shur B. D. Glycosyltransferases as cell adhesion molecules. Curr Opin Cell Biol. 1993 Oct;5(5):854–863. doi: 10.1016/0955-0674(93)90035-o. [DOI] [PubMed] [Google Scholar]
- Strous G. J., van Kerkhof P., Fallon R. J., Schwartz A. L. Golgi galactosyltransferase contains serine-linked phosphate. Eur J Biochem. 1987 Dec 1;169(2):307–311. doi: 10.1111/j.1432-1033.1987.tb13613.x. [DOI] [PubMed] [Google Scholar]
- Sumar N., Bodman K. B., Rademacher T. W., Dwek R. A., Williams P., Parekh R. B., Edge J., Rook G. A., Isenberg D. A., Hay F. C. Analysis of glycosylation changes in IgG using lectins. J Immunol Methods. 1990 Jul 20;131(1):127–136. doi: 10.1016/0022-1759(90)90242-n. [DOI] [PubMed] [Google Scholar]
- Thompson S. J., Hitsumoto Y., Zhang Y. W., Rook G. A., Elson C. J. Agalactosyl IgG in pristane-induced arthritis. Pregnancy affects the incidence and severity of arthritis and the glycosylation status of IgG. Clin Exp Immunol. 1992 Sep;89(3):434–438. doi: 10.1111/j.1365-2249.1992.tb06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya N., Endo T., Matsuta K., Yoshinoya S., Aikawa T., Kosuge E., Takeuchi F., Miyamoto T., Kobata A. Effects of galactose depletion from oligosaccharide chains on immunological activities of human IgG. J Rheumatol. 1989 Mar;16(3):285–290. [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Wilson I. B., Platt F. M., Isenberg D. A., Rademacher T. W. Aberrant control of galactosyltransferase in peripheral B lymphocytes and Epstein-Barr virus transformed B lymphoblasts from patients with rheumatoid arthritis. J Rheumatol. 1993 Aug;20(8):1282–1287. [PubMed] [Google Scholar]
- Yamashita Y., Imai Y., Osawa T. Poly[N-acetyl-lactosamine]-type sugar chains in CD45 antigens of abnormal T cells of lpr mice are different from those of normal T cells and B cells. Mol Immunol. 1989 Sep;26(9):905–913. doi: 10.1016/0161-5890(89)90147-8. [DOI] [PubMed] [Google Scholar]
- Young A., Sumar N., Bodman K., Goyal S., Sinclair H., Roitt I., Isenberg D. Agalactosyl IgG: an aid to differential diagnosis in early synovitis. Arthritis Rheum. 1991 Nov;34(11):1425–1429. doi: 10.1002/art.1780341113. [DOI] [PubMed] [Google Scholar]
- van Zeben D., Hazes J. M., Zwinderman A. H., Vandenbroucke J. P., Breedveld F. C. Factors predicting outcome of rheumatoid arthritis: results of a followup study. J Rheumatol. 1993 Aug;20(8):1288–1296. [PubMed] [Google Scholar]