Abstract
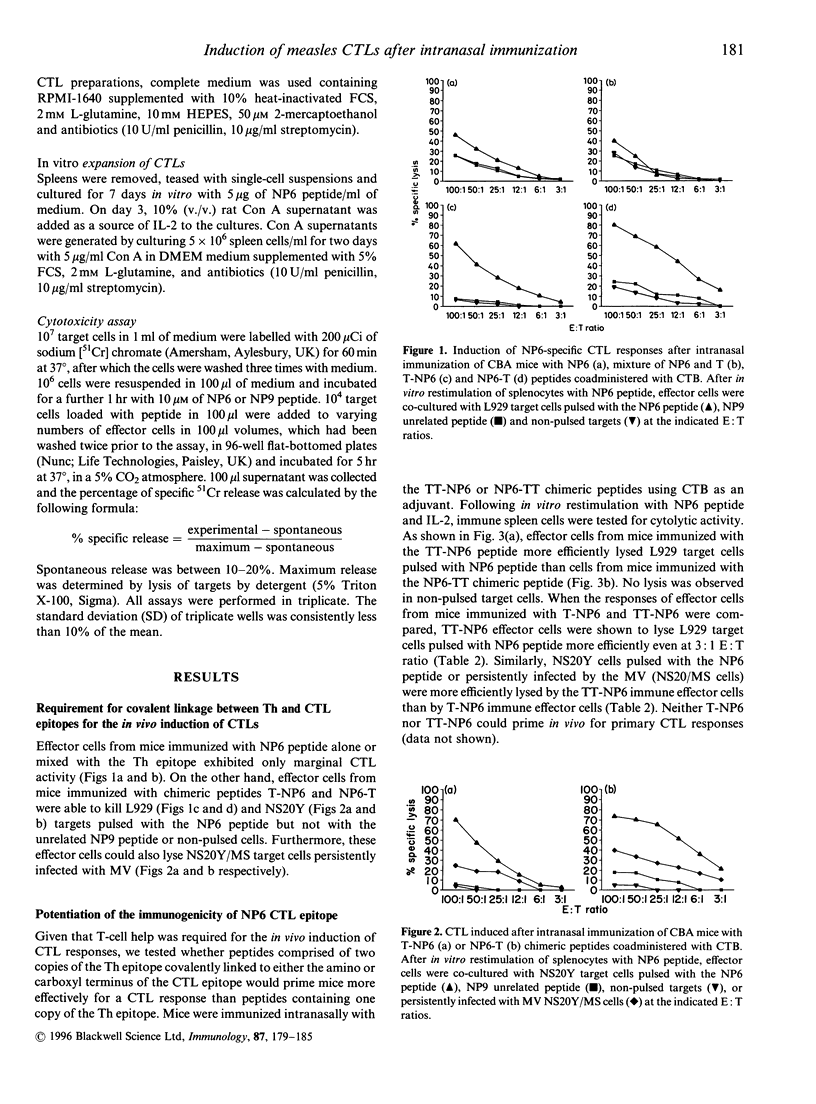
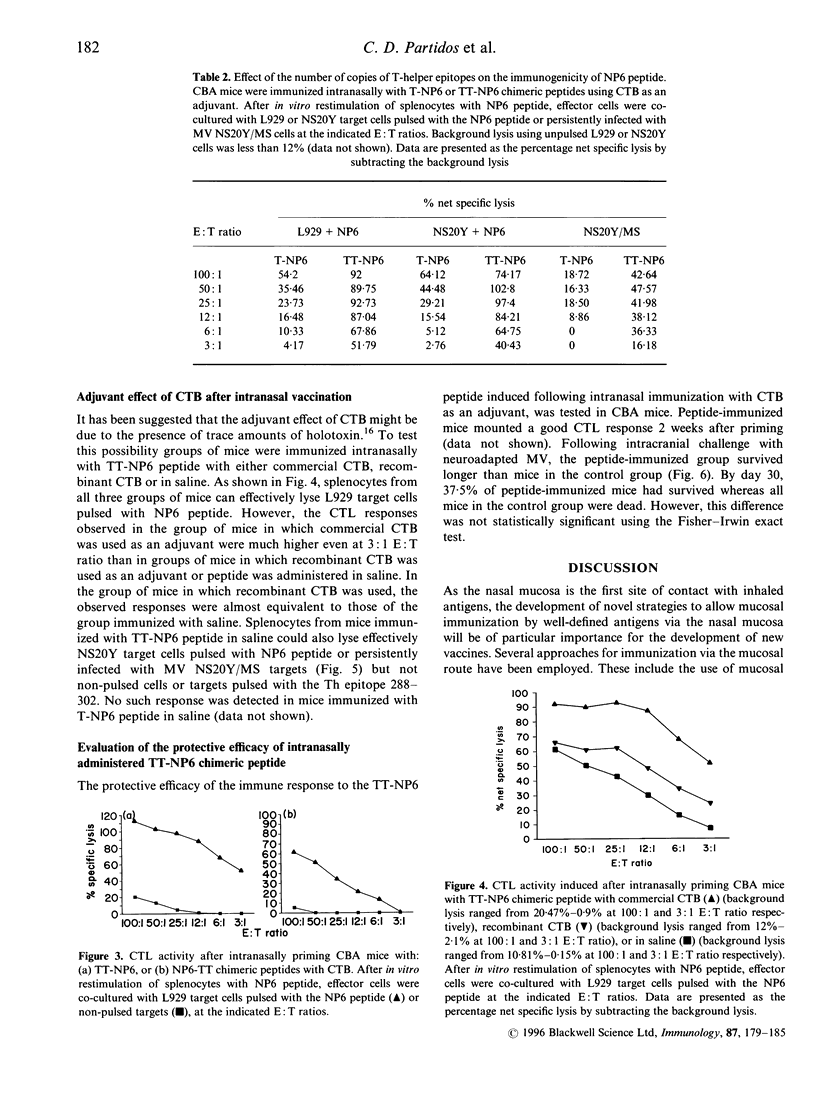
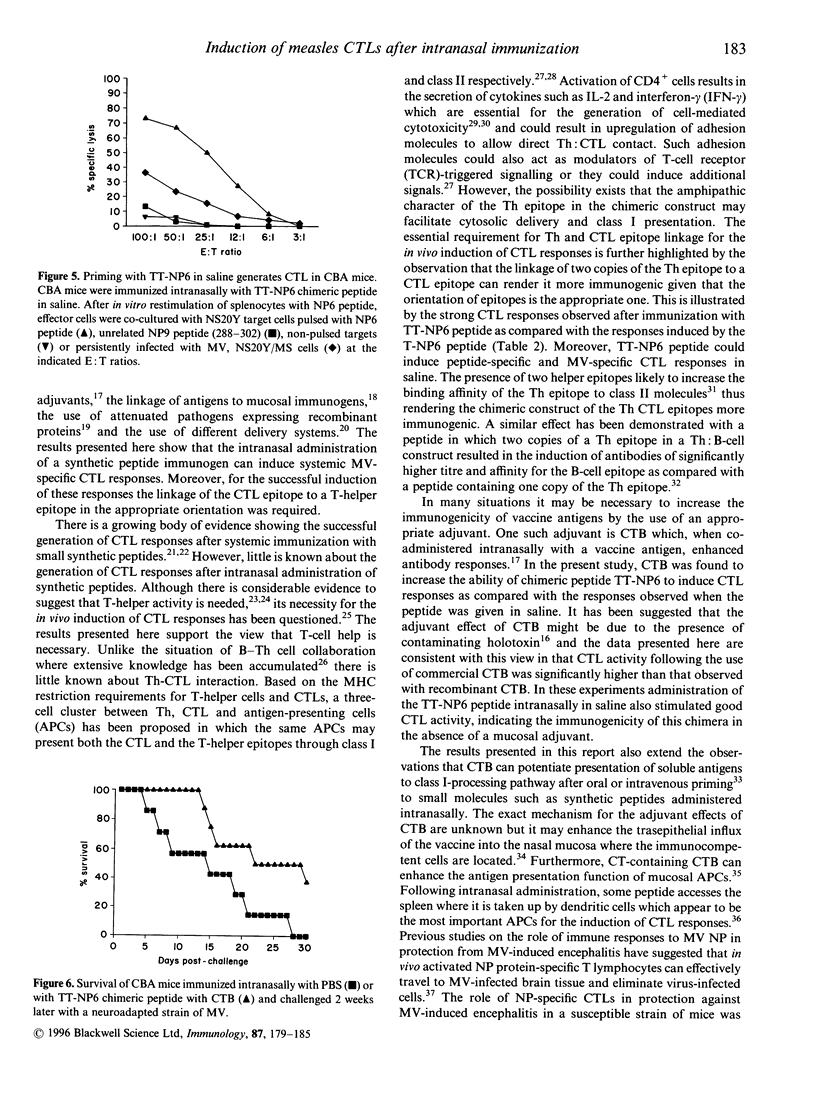
We have investigated the structural requirements for the induction of cytotoxic T-cell responses (CTL) in vivo after intranasal immunization with an immunodominant CTL epitope from the nucleoprotein of measles virus (MV). For the induction of CTL responses, covalent linkage of the CTL epitope to a helper T-cell epitope was required and the orientation of the epitopes influenced the immunogenicity of the CTL epitope. The presence of two copies as compared with one copy of a T-helper epitope, rendered the CTL epitope more immunogenic and resulted in the in vivo induction of MV-specific CTLs without the need for an adjuvant. The role of CTL responses to this epitope in protection after intranasal administration was evaluated in a mouse model against challenge with a neuroadapted strain of MV. Although a decreased mortality in the peptide immunized compared with that in unimmunized mice was observed, the protection achieved was not significant. These findings highlight the importance of the rational design of synthetic immunogens for the induction of CTL responses and the potential of the intranasal route for immunization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. L., Newman F. K., Maassab H. F., Belshe R. B. Evaluation of a cold-adapted influenza B/Texas/84 reassortant virus (CRB-87) vaccine in young children. J Clin Microbiol. 1992 Sep;30(9):2230–2234. doi: 10.1128/jcm.30.9.2230-2234.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankamp B., Brinckmann U. G., Reich A., Niewiesk S., ter Meulen V., Liebert U. G. Measles virus nucleocapsid protein protects rats from encephalitis. J Virol. 1991 Apr;65(4):1695–1700. doi: 10.1128/jvi.65.4.1695-1700.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauverger P., Chadwick J., Buckland R., Wild T. F. Serotype-specific and canine distemper virus cross-reactive H-2Kk-restricted cytotoxic T lymphocyte epitopes in the measles virus nucleoprotein. Virology. 1994 Aug 15;203(1):172–177. doi: 10.1006/viro.1994.1470. [DOI] [PubMed] [Google Scholar]
- Bowen J. C., Nair S. K., Reddy R., Rouse B. T. Cholera toxin acts as a potent adjuvant for the induction of cytotoxic T-lymphocyte responses with non-replicating antigens. Immunology. 1994 Mar;81(3):338–342. [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Holmes K. L., Hügin A., Frederickson T. N., Morse H. C., 3rd Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature. 1987 Jul 2;328(6125):77–79. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- Deres K., Schild H., Wiesmüller K. H., Jung G., Rammensee H. G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989 Nov 30;342(6249):561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- Erard F., Corthesy P., Nabholz M., Lowenthal J. W., Zaech P., Plaetinck G., MacDonald H. R. Interleukin 2 is both necessary and sufficient for the growth and differentiation of lectin-stimulated cytolytic T lymphocyte precursors. J Immunol. 1985 Mar;134(3):1644–1652. [PubMed] [Google Scholar]
- Fayolle C., Deriaud E., Leclerc C. In vivo induction of cytotoxic T cell response by a free synthetic peptide requires CD4+ T cell help. J Immunol. 1991 Dec 15;147(12):4069–4073. [PubMed] [Google Scholar]
- GOOD R. A., ZAK S. J. Disturbances in gamma globulin synthesis as experiments of nature. Pediatrics. 1956 Jul;18(1):109–149. [PubMed] [Google Scholar]
- Gizurarson S., Tamura S., Aizawa C., Kurata T. Stimulation of the transepithelial flux of influenza HA vaccine by cholera toxin B subunit. Vaccine. 1992;10(2):101–106. doi: 10.1016/0264-410x(92)90025-f. [DOI] [PubMed] [Google Scholar]
- Gopas J., Itzhaky D., Segev Y., Salzberg S., Trink B., Isakov N., Rager-Zisman B. Persistent measles virus infection enhances major histocompatibility complex class I expression and immunogenicity of murine neuroblastoma cells. Cancer Immunol Immunother. 1992;34(5):313–320. doi: 10.1007/BF01741552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishioka G. Y., Colon S., Miles C., Grey H. M., Chesnut R. W. Induction of class I MHC-restricted, peptide-specific cytolytic T lymphocytes by peptide priming in vivo. J Immunol. 1989 Aug 15;143(4):1094–1100. [PubMed] [Google Scholar]
- Kreth H. W., ter Meulen V., Eckert G. Demonstration of HLA restricted killer cells in patients with acute measles. Med Microbiol Immunol. 1979 Jan 24;165(4):203–214. doi: 10.1007/BF02152920. [DOI] [PubMed] [Google Scholar]
- Langermann S., Palaszynski S., Sadziene A., Stover C. K., Koenig S. Systemic and mucosal immunity induced by BCG vector expressing outer-surface protein A of Borrelia burgdorferi. Nature. 1994 Dec 8;372(6506):552–555. doi: 10.1038/372552a0. [DOI] [PubMed] [Google Scholar]
- Lucas C. J., Biddison W. E., Nelson D. L., Shaw S. Killing of measles virus-infected cells by human cytotoxic T cells. Infect Immun. 1982 Oct;38(1):226–232. doi: 10.1128/iai.38.1.226-232.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Donachie A. M. ISCOMS--a novel strategy for mucosal immunization? Immunol Today. 1991 Nov;12(11):383–385. doi: 10.1016/0167-5699(91)90133-E. [DOI] [PubMed] [Google Scholar]
- Nair S., Buiting A. M., Rouse R. J., Van Rooijen N., Huang L., Rouse B. T. Role of macrophages and dendritic cells in primary cytotoxic T lymphocyte responses. Int Immunol. 1995 Apr;7(4):679–688. doi: 10.1093/intimm/7.4.679. [DOI] [PubMed] [Google Scholar]
- Obeid O. E., Partidos C. D., Howard C. R., Steward M. W. Protection against morbillivirus-induced encephalitis by immunization with a rationally designed synthetic peptide vaccine containing B- and T-cell epitopes from the fusion protein of measles virus. J Virol. 1995 Mar;69(3):1420–1428. doi: 10.1128/jvi.69.3.1420-1428.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partidos C. D., Stanley C. M., Steward M. W. Immune responses in mice following immunization with chimeric synthetic peptides representing B and T cell epitopes of measles virus proteins. J Gen Virol. 1991 Jun;72(Pt 6):1293–1299. doi: 10.1099/0022-1317-72-6-1293. [DOI] [PubMed] [Google Scholar]
- Partidos C. D., Steward M. W. Prediction and identification of a T cell epitope in the fusion protein of measles virus immunodominant in mice and humans. J Gen Virol. 1990 Sep;71(Pt 9):2099–2105. doi: 10.1099/0022-1317-71-9-2099. [DOI] [PubMed] [Google Scholar]
- Partidos C., Stanley C., Steward M. The influence of orientation and number of copies of T and B cell epitopes on the specificity and affinity of antibodies induced by chimeric peptides. Eur J Immunol. 1992 Oct;22(10):2675–2680. doi: 10.1002/eji.1830221030. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Egan J. E., Kress Y., Bloom B. R. Isolation of cold-sensitive mutants of measles virus from persistently infected murine neuroblastoma cells. J Virol. 1984 Sep;51(3):845–855. doi: 10.1128/jvi.51.3.845-855.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Gamble S., Rothstein L. Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science. 1990 Aug 24;249(4971):918–921. doi: 10.1126/science.2392683. [DOI] [PubMed] [Google Scholar]
- Sabin A. B., Flores Arechiga A., Fernández de Castro J., Albrecht P., Sever J. L., Shekarchi I. Successful immunization of infants with and without maternal antibody by aerosolized measles vaccine. II. Vaccine comparisons and evidence for multiple antibody response. JAMA. 1984 May 11;251(18):2363–2371. [PubMed] [Google Scholar]
- Simon M. M., Hochgeschwender U., Brugger U., Landolfo S. Monoclonal antibodies to interferon-gamma inhibit interleukin 2-dependent induction of growth and maturation in lectin/antigen-reactive cytolytic T lymphocyte precursors. J Immunol. 1986 Apr 15;136(8):2755–2762. [PubMed] [Google Scholar]
- Singer A., Hodes R. J. Mechanisms of T cell-B cell interaction. Annu Rev Immunol. 1983;1:211–241. doi: 10.1146/annurev.iy.01.040183.001235. [DOI] [PubMed] [Google Scholar]
- Stuhler G., Walden P. Collaboration of helper and cytotoxic T lymphocytes. Eur J Immunol. 1993 Sep;23(9):2279–2286. doi: 10.1002/eji.1830230934. [DOI] [PubMed] [Google Scholar]
- Tamura S., Ishihara K., Miyata K., Aizawa C., Kurata T. Mechanism of enhancement of the immune responses to influenza vaccine with cholera toxin B subunit and a trace amount of holotoxin. Vaccine. 1995 Mar;13(4):339–341. doi: 10.1016/0264-410x(95)98253-7. [DOI] [PubMed] [Google Scholar]
- Tamura S., Yamanaka A., Shimohara M., Tomita T., Komase K., Tsuda Y., Suzuki Y., Nagamine T., Kawahara K., Danbara H. Synergistic action of cholera toxin B subunit (and Escherichia coli heat-labile toxin B subunit) and a trace amount of cholera whole toxin as an adjuvant for nasal influenza vaccine. Vaccine. 1994 Apr;12(5):419–426. doi: 10.1016/0264-410x(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Treanor J. J., Mattison H. R., Dumyati G., Yinnon A., Erb S., O'Brien D., Dolin R., Betts R. F. Protective efficacy of combined live intranasal and inactivated influenza A virus vaccines in the elderly. Ann Intern Med. 1992 Oct 15;117(8):625–633. doi: 10.7326/0003-4819-117-8-625. [DOI] [PubMed] [Google Scholar]
- UytdeHaag F. G., van Binnendijk R. S., Kenter M. J., Osterhaus A. D. Cytotoxic T lymphocyte responses against measles virus. Curr Top Microbiol Immunol. 1994;189:151–167. doi: 10.1007/978-3-642-78530-6_9. [DOI] [PubMed] [Google Scholar]
- Valero M. V., Amador L. R., Galindo C., Figueroa J., Bello M. S., Murillo L. A., Mora A. L., Patarroyo G., Rocha C. L., Rojas M. Vaccination with SPf66, a chemically synthesised vaccine, against Plasmodium falciparum malaria in Colombia. Lancet. 1993 Mar 20;341(8847):705–710. doi: 10.1016/0140-6736(93)90483-w. [DOI] [PubMed] [Google Scholar]
- Widmann C., Romero P., Maryanski J. L., Corradin G., Valmori D. T helper epitopes enhance the cytotoxic response of mice immunized with MHC class I-restricted malaria peptides. J Immunol Methods. 1992 Oct 19;155(1):95–99. doi: 10.1016/0022-1759(92)90275-x. [DOI] [PubMed] [Google Scholar]


