Abstract
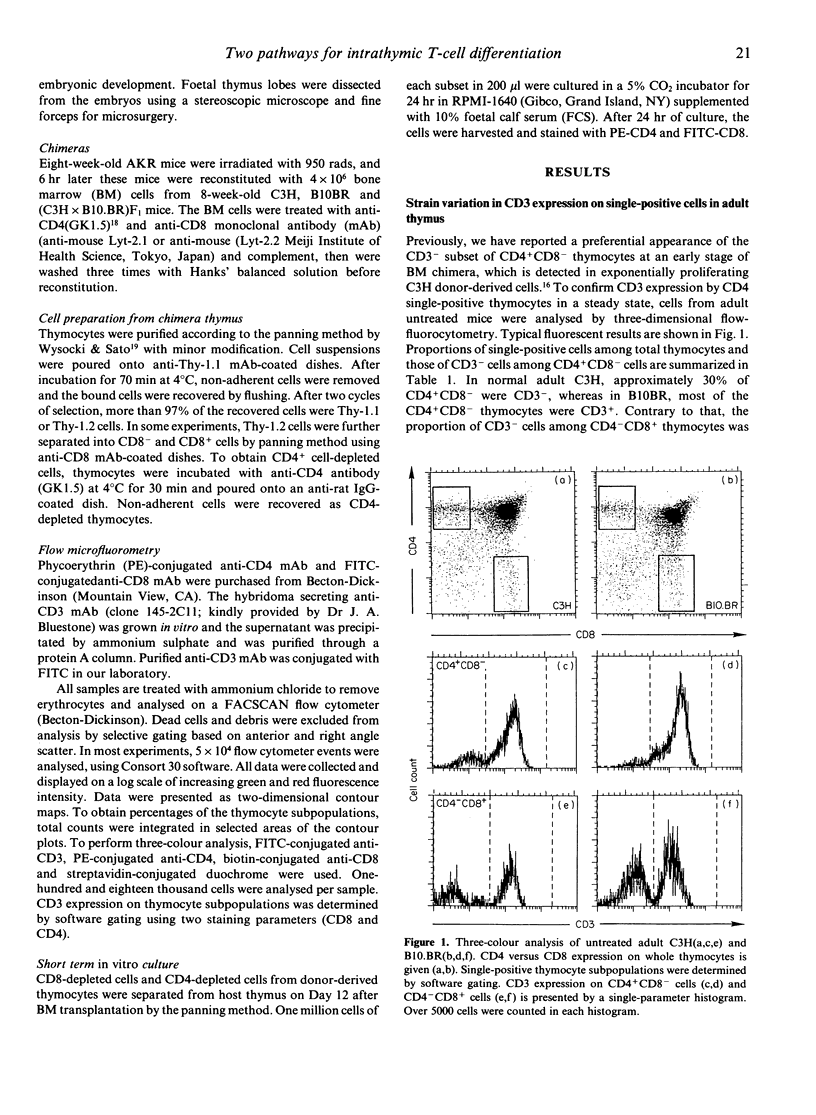
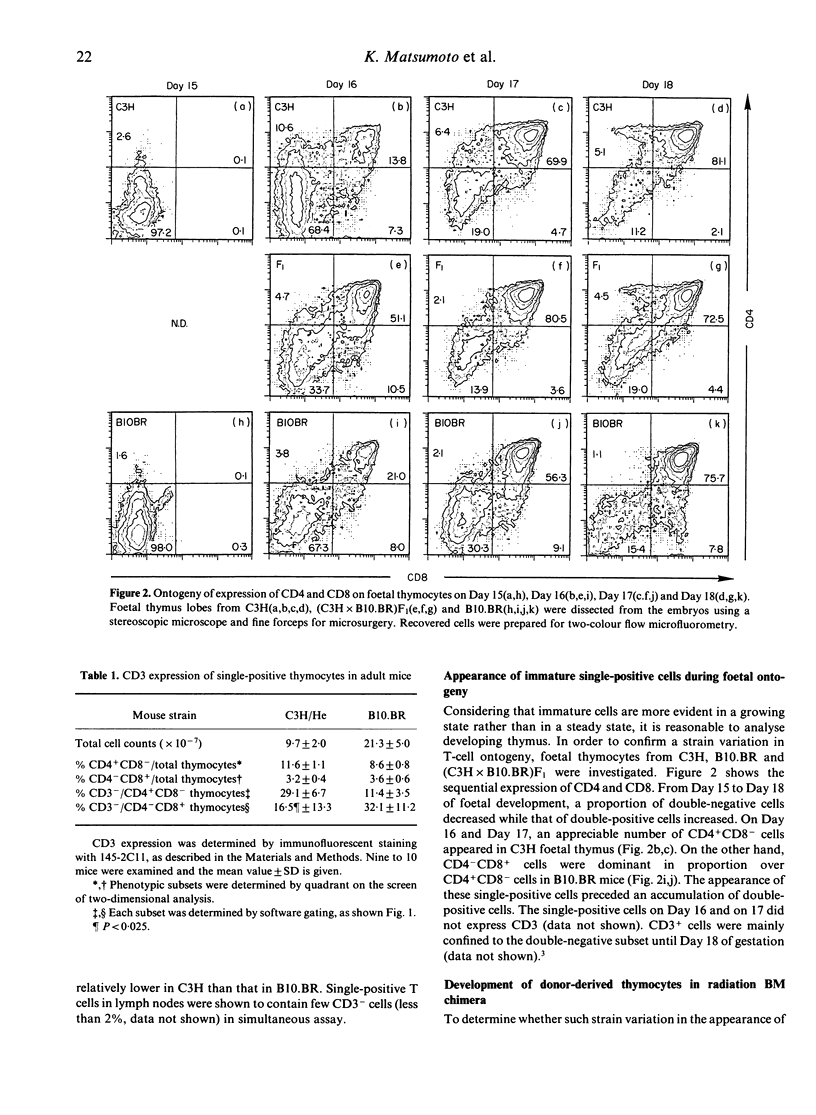
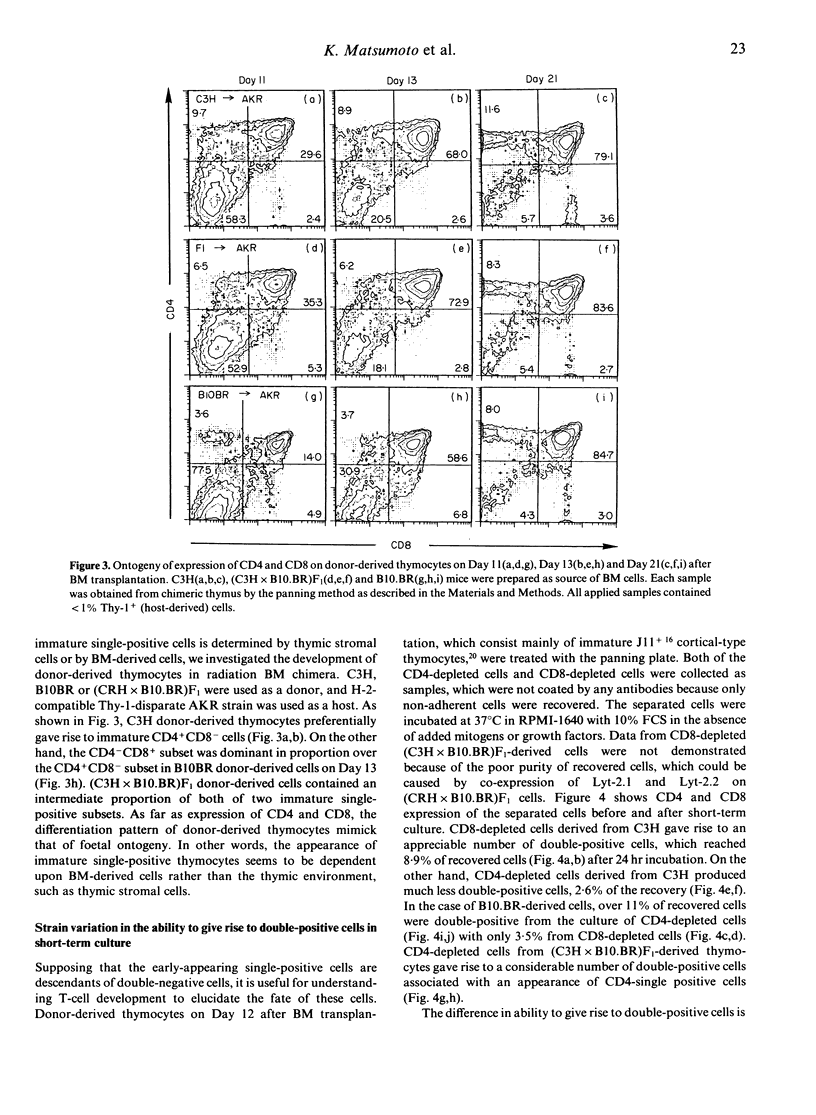
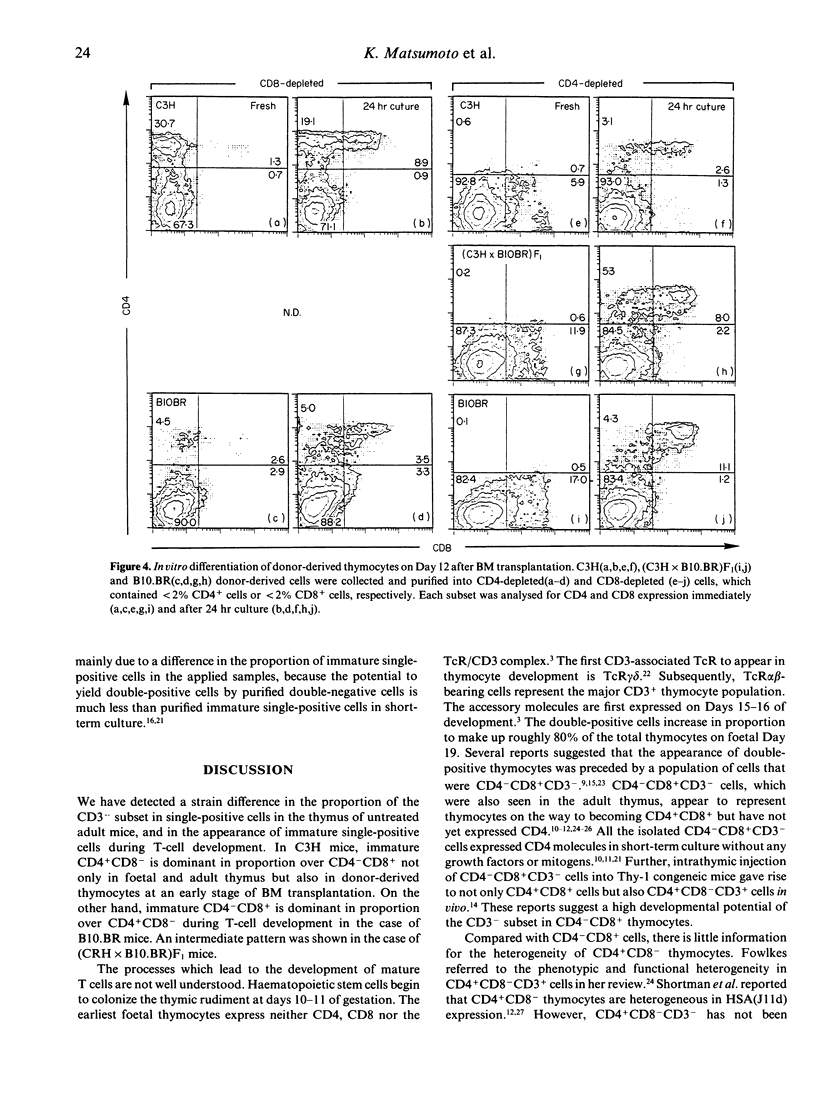
Murine thymocytes are divided into four major populations on the basis of expression of CD4 and CD8 antigens. The bulk of evidence favours the view that CD4-CD8- cells can develop into CD4-CD8+ and CD4+CD8- cells via the CD4+CD8+ stage in the thymus. However, CD4-CD8+ and CD4+CD8- thymocyte subsets contain not only CD3+ mature cells but also CD3- immature cells, which seem to be intermediate cells between CD4-CD8- and CD4+CD8+ cells. Here we demonstrate mouse strain differences in the proportion of immature single-positive thymocyte subsets in thymus at the steady or developing state. In C3H mice, immature CD4+CD8- is dominant in proportion over CD4-CD8+ in foetal thymus and in donor-derived thymocytes at an early stage of bone marrow transplantation. On the other hand, immature CD4-CD8+ is dominant over CD4+CD8- during T-cell development in the case of B10.BR mice. An intermediate pattern was shown in the case of F1 mice. Both of these immature single-positive subsets gave rise to double-positive cells after 24 hr culture. These results suggest that there exist two distinct differential pathways; one is from CD4-CD8- cells to CD4+CD8+ cells via CD4-CD8+ cells, and another is via CD4+CD8- cells, and that an application of the 'CD8 pathway' or 'CD4 pathway' seems to be genetically destined by BM-derived cells but not by thymic stromal cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bluestone J. A., Pardoll D., Sharrow S. O., Fowlkes B. J. Characterization of murine thymocytes with CD3-associated T-cell receptor structures. Nature. 1987 Mar 5;326(6108):82–84. doi: 10.1038/326082a0. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Dialynas D. P., Fitch F. W., MacDonald H. R. Precursors of T cell growth factor producing cells in the thymus: ontogeny, frequency, and quantitative recovery in a subpopulation of phenotypically mature thymocytes defined by monoclonal antibody GK-1.5. J Exp Med. 1983 Nov 1;158(5):1654–1671. doi: 10.1084/jem.158.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceredig R. Differentiation potential of 14-day fetal mouse thymocytes in organ culture. Analysis of CD4/CD8-defined single-positive and double-negative cells. J Immunol. 1988 Jul 15;141(2):355–362. [PubMed] [Google Scholar]
- Crispe I. N., Bevan M. J. Expression and functional significance of the J11d marker on mouse thymocytes. J Immunol. 1987 Apr 1;138(7):2013–2018. [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Fowlkes B. J., Edison L., Mathieson B. J., Chused T. M. Early T lymphocytes. Differentiation in vivo of adult intrathymic precursor cells. J Exp Med. 1985 Sep 1;162(3):802–822. doi: 10.1084/jem.162.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes B. J., Pardoll D. M. Molecular and cellular events of T cell development. Adv Immunol. 1989;44:207–264. doi: 10.1016/s0065-2776(08)60643-4. [DOI] [PubMed] [Google Scholar]
- Fowlkes B. J., Schwartz R. H., Pardoll D. M. Deletion of self-reactive thymocytes occurs at a CD4+8+ precursor stage. Nature. 1988 Aug 18;334(6183):620–623. doi: 10.1038/334620a0. [DOI] [PubMed] [Google Scholar]
- Guidos C. J., Weissman I. L., Adkins B. Intrathymic maturation of murine T lymphocytes from CD8+ precursors. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7542–7546. doi: 10.1073/pnas.86.19.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havran W. L., Poenie M., Kimura J., Tsien R., Weiss A., Allison J. P. Expression and function of the CD3-antigen receptor on murine CD4+8+ thymocytes. Nature. 1987 Nov 12;330(6144):170–173. doi: 10.1038/330170a0. [DOI] [PubMed] [Google Scholar]
- Hirokawa K., Sado T., Kubo S., Kamisaku H., Hitomi K., Utsuyama M. Intrathymic T cell differentiation in radiation bone marrow chimeras and its role in T cell emigration to the spleen. An immunohistochemical study. J Immunol. 1985 Jun;134(6):3615–3624. [PubMed] [Google Scholar]
- Hugo P., Waanders G. A., Scollay R., Shortman K., Boyd R. L. Ontogeny of a novel CD4+CD8-CD3- thymocyte subpopulation: a comparison with CD4- CD8+ CD3- thymocytes. Int Immunol. 1990;2(3):209–218. doi: 10.1093/intimm/2.3.209. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Leiserson W., Von Boehmer H. Differentiation of thymocytes in fetal organ culture: analysis of phenotypic changes accompanying the appearance of cytolytic and interleukin 2-producing cells. J Immunol. 1984 Sep;133(3):1117–1123. [PubMed] [Google Scholar]
- MacDonald H. R., Budd R. C., Howe R. C. A CD3- subset of CD4-8+ thymocytes: a rapidly cycling intermediate in the generation of CD4+8+ cells. Eur J Immunol. 1988 Apr;18(4):519–523. doi: 10.1002/eji.1830180405. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Hengartner H., Pedrazzini T. Intrathymic deletion of self-reactive cells prevented by neonatal anti-CD4 antibody treatment. Nature. 1988 Sep 8;335(6186):174–176. doi: 10.1038/335174a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Yoshikai Y., Matsuzaki G., Asano T., Nomoto K. A novel CD3-J11d+ subset of CD4+CD8- cells repopulating thymus in radiation bone marrow chimeras. Eur J Immunol. 1989 Jul;19(7):1203–1207. doi: 10.1002/eji.1830190708. [DOI] [PubMed] [Google Scholar]
- Nikolić-Zugić J., Moore M. W., Bevan M. J. Characterization of the subset of immature thymocytes which can undergo rapid in vitro differentiation. Eur J Immunol. 1989 Apr;19(4):649–653. doi: 10.1002/eji.1830190412. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M., Fowlkes B. J., Bluestone J. A., Kruisbeek A., Maloy W. L., Coligan J. E., Schwartz R. H. Differential expression of two distinct T-cell receptors during thymocyte development. Nature. 1987 Mar 5;326(6108):79–81. doi: 10.1038/326079a0. [DOI] [PubMed] [Google Scholar]
- Paterson D. J., Williams A. F. An intermediate cell in thymocyte differentiation that expresses CD8 but not CD4 antigen. J Exp Med. 1987 Nov 1;166(5):1603–1608. doi: 10.1084/jem.166.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penit C., Vasseur F. Cell proliferation and differentiation in the fetal and early postnatal mouse thymus. J Immunol. 1989 May 15;142(10):3369–3377. [PubMed] [Google Scholar]
- Scollay R., Shortman K. Identification of early stages of T lymphocyte development in the thymus cortex and medulla. J Immunol. 1985 Jun;134(6):3632–3642. [PubMed] [Google Scholar]
- Shortman K., Wilson A., Egerton M., Pearse M., Scollay R. Immature CD4- CD8+ murine thymocytes. Cell Immunol. 1988 May;113(2):462–479. doi: 10.1016/0008-8749(88)90042-1. [DOI] [PubMed] [Google Scholar]
- Wilson A., Day L. M., Scollay R., Shortman K. Subpopulations of mature murine thymocytes: properties of CD4-CD8+ and CD4+CD8- thymocytes lacking the heat-stable antigen. Cell Immunol. 1988 Dec;117(2):312–326. doi: 10.1016/0008-8749(88)90121-9. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]


