Abstract
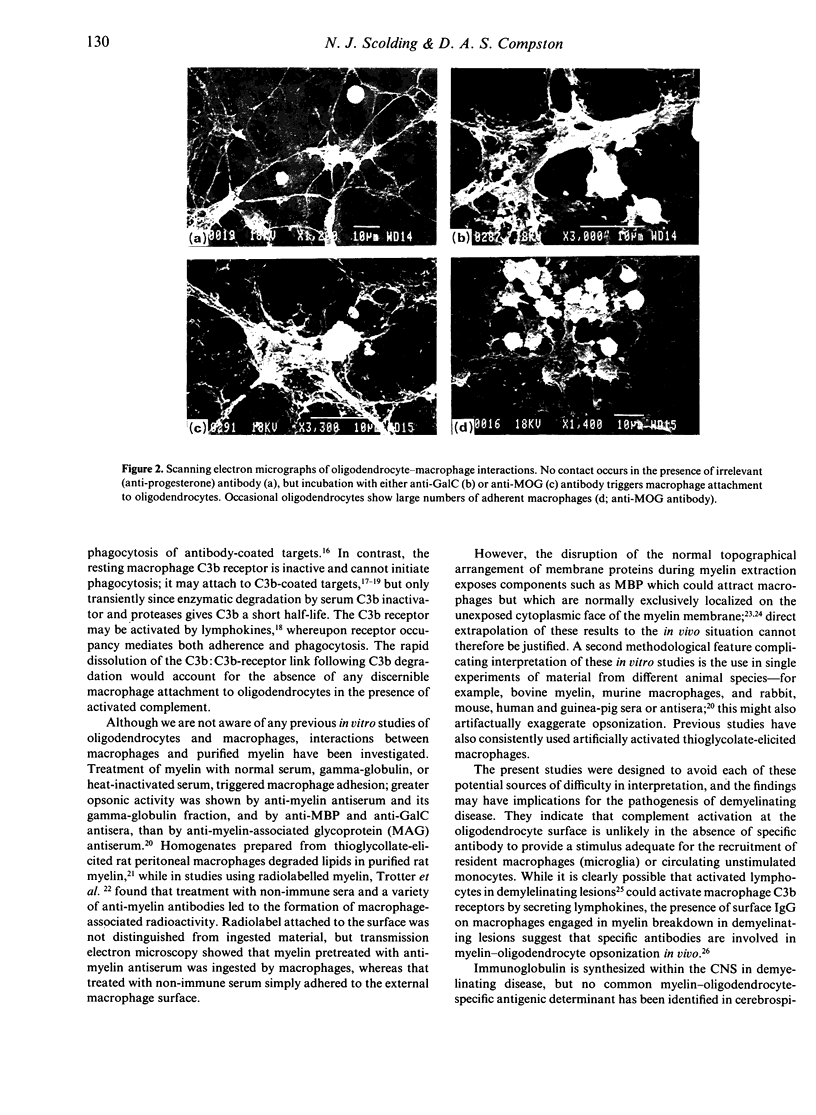
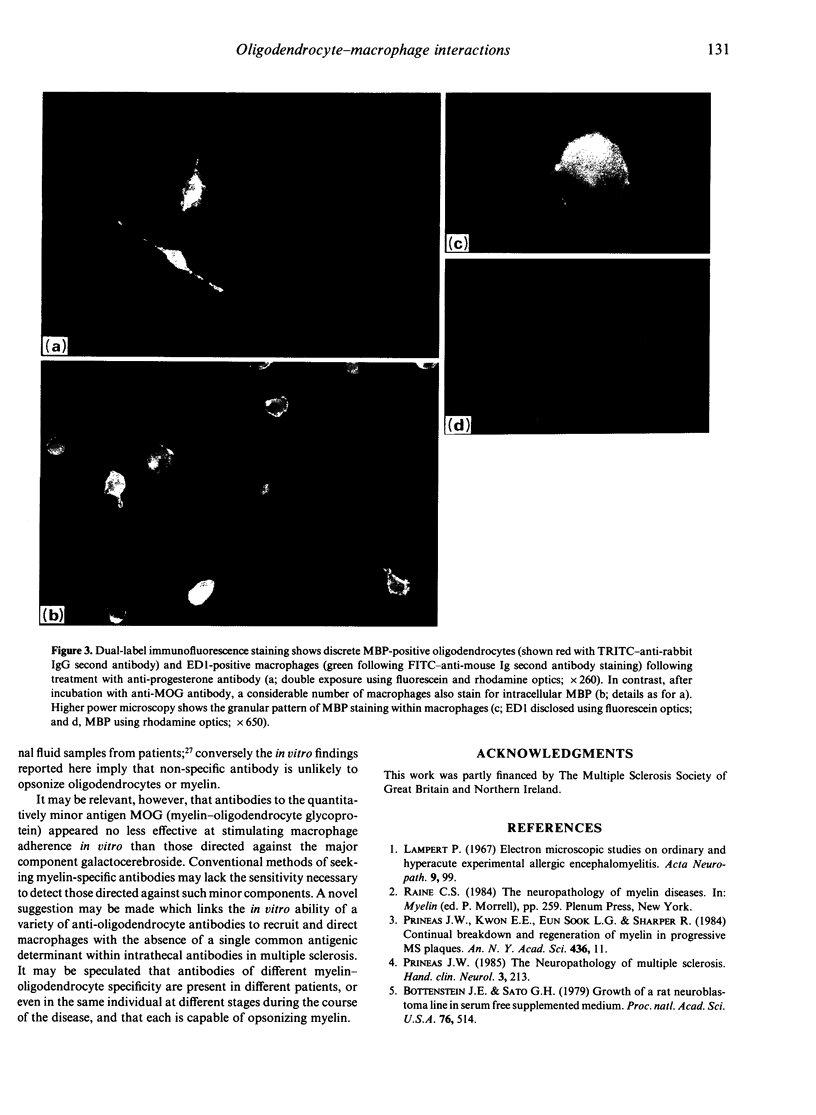
The final pathway of myelin destruction in immune-mediated demyelination is phagocytosis by macrophages. As part of a systematic study of mechanisms of myelin-oligodendrocyte injury, we have used an in vitro approach to investigate interactions between rat oligodendrocytes and macrophages in order to identify the conditions under which macrophages adhere to and damage oligodendrocytes. No adherence was seen when macrophages alone were co-cultured with homologous oligodendrocytes. However, macrophage attachment to oligodendrocytes was triggered not only by antibody to the major cell-surface component galactocerebroside, but also by antibody to the quantitatively minor antigen, myelin-oligodendrocyte glycoprotein; immunocytochemical observations suggested that phagocytosis of myelin antigen also occurred. No such changes were seen in the presence of an irrelevant (anti-progesterone) antibody, or in the presence of activated complement. These results emphasize that a variety of antibodies, including those to minor myelin-oligodendrocyte antigens, may play a significant role in the development of demyelinated lesions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Goldenberg P. Z., Kwon E. E., Benjamins J. A., Whitaker J. N., Quarles R. H., Prineas J. W. Opsonization of normal myelin by anti-myelin antibodies and normal serum. J Neuroimmunol. 1989 Jul;23(2):157–166. doi: 10.1016/0165-5728(89)90035-0. [DOI] [PubMed] [Google Scholar]
- Hofman F. M., von Hanwehr R. I., Dinarello C. A., Mizel S. B., Hinton D., Merrill J. E. Immunoregulatory molecules and IL 2 receptors identified in multiple sclerosis brain. J Immunol. 1986 May 1;136(9):3239–3245. [PubMed] [Google Scholar]
- Lampert P. Electron microscopic studies on ordinary and hyperacute experimental allergic encephalomyelitis. Acta Neuropathol. 1967 Oct 20;9(2):99–126. doi: 10.1007/BF00691436. [DOI] [PubMed] [Google Scholar]
- Linnington C., Webb M., Woodhams P. L. A novel myelin-associated glycoprotein defined by a mouse monoclonal antibody. J Neuroimmunol. 1984 Sep-Oct;6(6):387–396. doi: 10.1016/0165-5728(84)90064-x. [DOI] [PubMed] [Google Scholar]
- Michl J., Pieczonka M. M., Unkeless J. C., Silverstein S. C. Effects of immobilized immune complexes on Fc- and complement-receptor function in resident and thioglycollate-elicited mouse peritoneal macrophages. J Exp Med. 1979 Sep 19;150(3):607–621. doi: 10.1084/jem.150.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omlin F. X., Webster H. D., Palkovits C. G., Cohen S. R. Immunocytochemical localization of basic protein in major dense line regions of central and peripheral myelin. J Cell Biol. 1982 Oct;95(1):242–248. doi: 10.1083/jcb.95.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prineas J. W., Graham J. S. Multiple sclerosis: capping of surface immunoglobulin G on macrophages engaged in myelin breakdown. Ann Neurol. 1981 Aug;10(2):149–158. doi: 10.1002/ana.410100205. [DOI] [PubMed] [Google Scholar]
- Prineas J. W., Kwon E. E., Cho E. S., Sharer L. R. Continual breakdown and regeneration of myelin in progressive multiple sclerosis plaques. Ann N Y Acad Sci. 1984;436:11–32. doi: 10.1111/j.1749-6632.1984.tb14773.x. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Robinson A. P., White T. M., Mason D. W. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986 Feb;57(2):239–247. [PMC free article] [PubMed] [Google Scholar]
- Scolding N. J., Morgan B. P., Houston A., Campbell A. K., Linington C., Compston D. A. Normal rat serum cytotoxicity against syngeneic oligodendrocytes. Complement activation and attack in the absence of anti-myelin antibodies. J Neurol Sci. 1989 Feb;89(2-3):289–300. doi: 10.1016/0022-510x(89)90030-0. [DOI] [PubMed] [Google Scholar]
- Sternberger N. H., Itoyama Y., Kies M. W., Webster H. D. Myelin basic protein demonstrated immunocytochemically in oligodendroglia prior to myelin sheath formation. Proc Natl Acad Sci U S A. 1978 May;75(5):2521–2524. doi: 10.1073/pnas.75.5.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter J., DeJong L. J., Smith M. E. Opsonization with antimyelin antibody increases the uptake and intracellular metabolism of myelin in inflammatory macrophages. J Neurochem. 1986 Sep;47(3):779–789. doi: 10.1111/j.1471-4159.1986.tb00679.x. [DOI] [PubMed] [Google Scholar]
- Trotter J., Smith M. E. The role of phospholipases from inflammatory macrophages in demyelination. Neurochem Res. 1986 Mar;11(3):349–361. doi: 10.1007/BF00965009. [DOI] [PubMed] [Google Scholar]
- Wren D. R., Noble M. Oligodendrocytes and oligodendrocyte/type-2 astrocyte progenitor cells of adult rats are specifically susceptible to the lytic effects of complement in absence of antibody. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9025–9029. doi: 10.1073/pnas.86.22.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]