Abstract
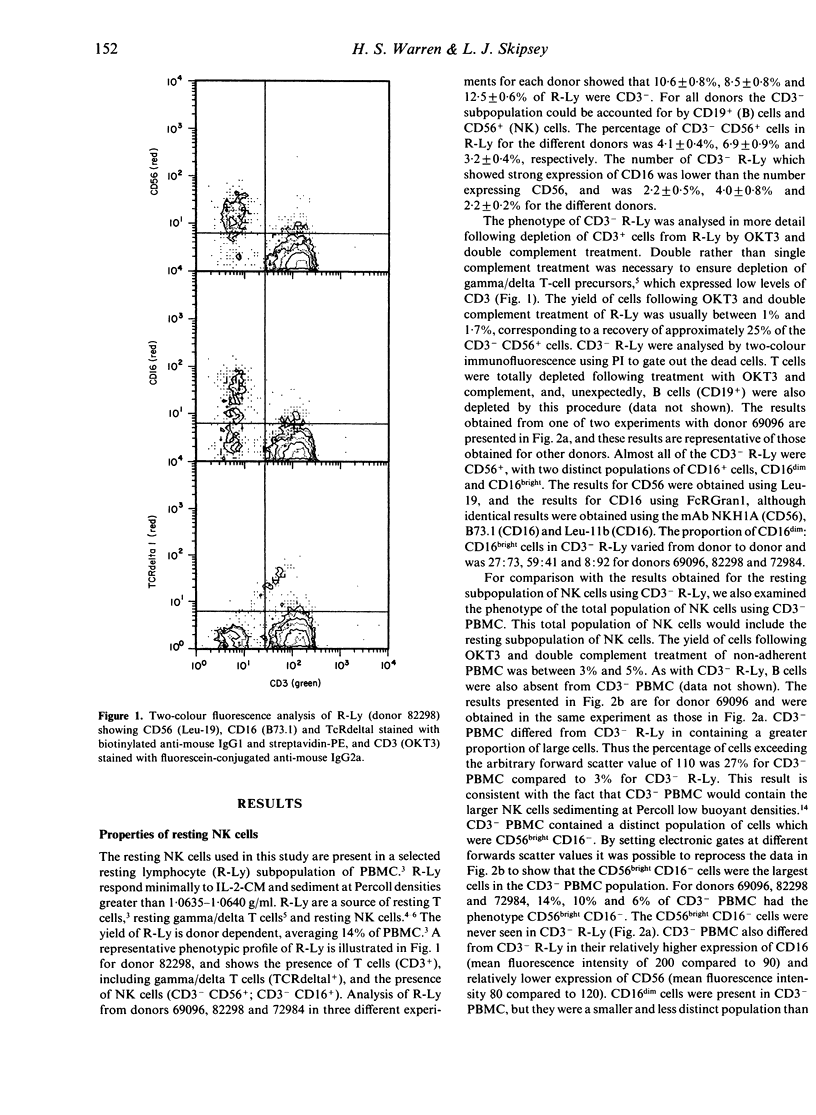
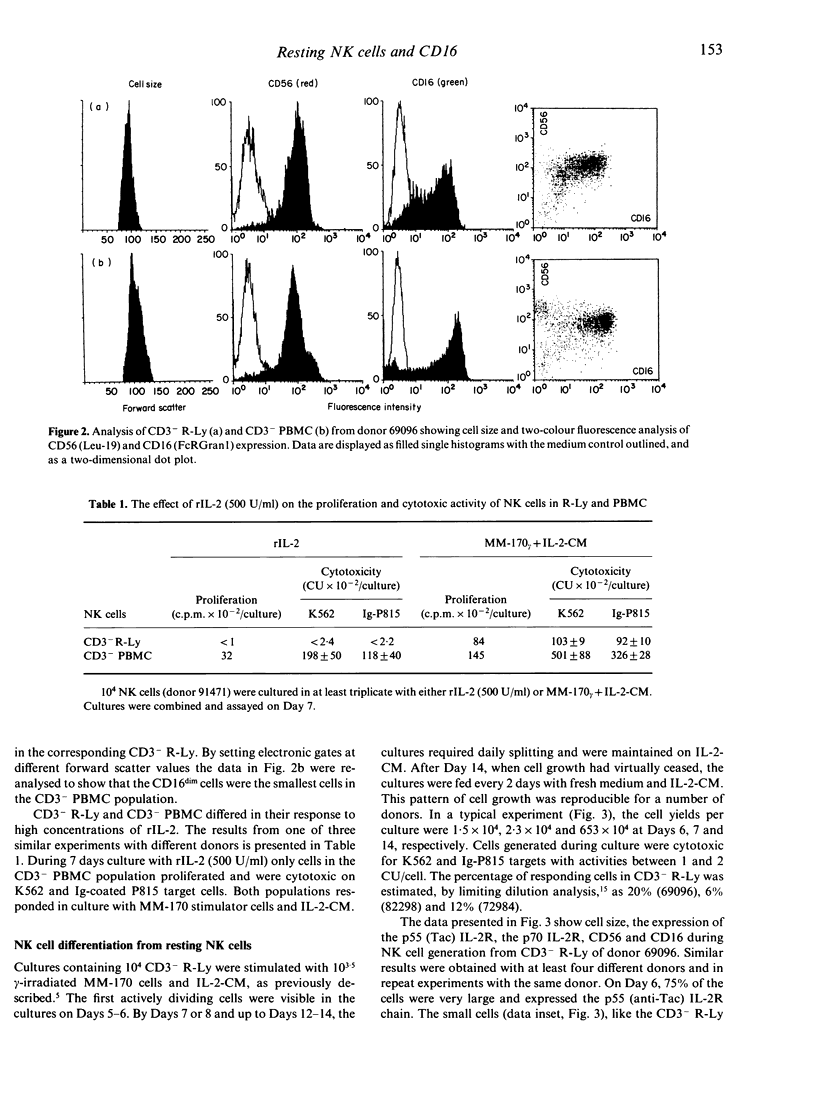
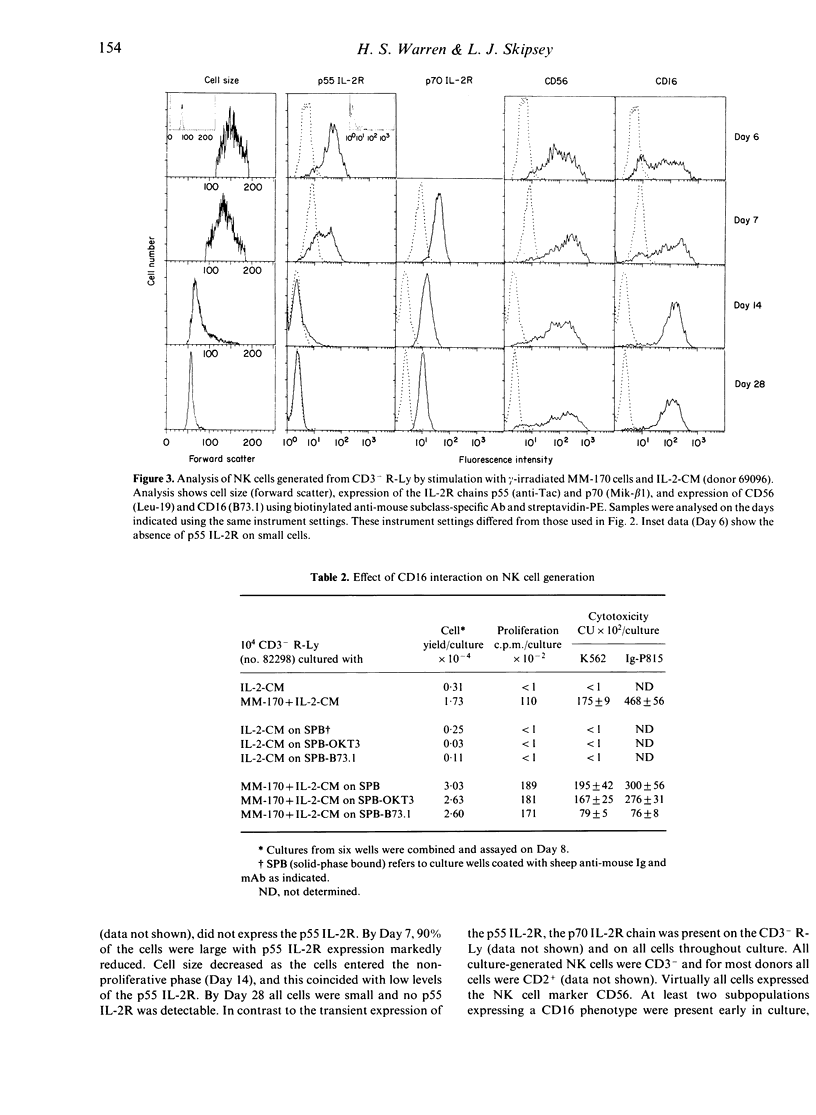
A subpopulation of human peripheral blood natural killer (NK) cells, defined by sedimentation at Percoll high buoyant densities (P greater than 1.0635-1.0640 g/ml) and unresponsiveness to interleukin-2 (IL-2), contained two distinct populations based on the intensity of CD16 (FcR gamma III) expression, namely CD16dim and CD16bright. This resting subpopulation of NK cells differed from the total population of peripheral blood NK cells, by containing a larger proportion of CD16dim cells, by the total absence of CD56bright CD16- cells, and by an inability to respond to high concentrations (500 U/ml) of rIL-2 despite the expression of an intermediate affinity (p70) IL-2R. Both CD16dim and CD16bright NK cells expressing high affinity IL-2R were initially generated following co-culture of resting NK cells with gamma-irradiated MM-170 cells and IL-2, but CD16bright NK cells became the dominant cell type later in culture. The CD16 molecule was not involved in the differentiation of resting NK cells since solid-phase-bound anti-CD16 monoclonal antibody neither enhanced nor inhibited NK cell generation. These studies demonstrate that the resting subpopulation of peripheral blood NK cells expresses a unique CD16 profile, that CD16 expression increases during NK cell generation, and that CD16 is not involved in the differentiation process.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Caligiuri M., Ritz J., Schlossman S. F. CD3-negative natural killer cells express zeta TCR as part of a novel molecular complex. Nature. 1989 Sep 14;341(6238):159–162. doi: 10.1038/341159a0. [DOI] [PubMed] [Google Scholar]
- Anegón I., Cuturi M. C., Trinchieri G., Perussia B. Interaction of Fc receptor (CD16) ligands induces transcription of interleukin 2 receptor (CD25) and lymphokine genes and expression of their products in human natural killer cells. J Exp Med. 1988 Feb 1;167(2):452–472. doi: 10.1084/jem.167.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Aribia M. H., Moiré N., Métivier D., Vaquero C., Lantz O., Olive D., Charpentier B., Senik A. IL-2 receptors on circulating natural killer cells and T lymphocytes. Similarity in number and affinity but difference in transmission of the proliferation signal. J Immunol. 1989 Jan 15;142(2):490–499. [PubMed] [Google Scholar]
- Caligiuri M. A., Zmuidzinas A., Manley T. J., Levine H., Smith K. A., Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med. 1990 May 1;171(5):1509–1526. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. T., Travis W. W., Koren H. S. Induction of activation antigens on human natural killer cells mediated through the Fc-gamma receptor. J Immunol. 1989 Oct 1;143(7):2401–2406. [PubMed] [Google Scholar]
- Janeway C. A. Natural killer cells: a primitive immune system. Nature. 1989 Sep 14;341(6238):108–108. doi: 10.1038/341108a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Phillips J. H., Testi R. Membrane anchoring and spontaneous release of CD16 (FcR III) by natural killer cells and granulocytes. Eur J Immunol. 1989 Apr;19(4):775–778. doi: 10.1002/eji.1830190431. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Ruitenberg J. J., Phillips J. H. Functional and biochemical analysis of CD16 antigen on natural killer cells and granulocytes. J Immunol. 1988 Nov 15;141(10):3478–3485. [PubMed] [Google Scholar]
- Lanier L. L., Yu G., Phillips J. H. Co-association of CD3 zeta with a receptor (CD16) for IgG Fc on human natural killer cells. Nature. 1989 Dec 14;342(6251):803–805. doi: 10.1038/342803a0. [DOI] [PubMed] [Google Scholar]
- Lindahl K. F., Wilson D. B. Histocompatibility antigen-activated cytotoxic T lymphocytes. II. Estimates of the frequency and specificity of precursors. J Exp Med. 1977 Mar 1;145(3):508–522. doi: 10.1084/jem.145.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London L., Perussia B., Trinchieri G. Induction of proliferation in vitro of resting human natural killer cells: IL 2 induces into cell cycle most peripheral blood NK cells, but only a minor subset of low density T cells. J Immunol. 1986 Dec 15;137(12):3845–3854. [PubMed] [Google Scholar]
- London L., Perussia B., Trinchieri G. Induction of proliferation in vitro of resting human natural killer cells: expression of surface activation antigens. J Immunol. 1985 Feb;134(2):718–727. [PubMed] [Google Scholar]
- Nagler A., Lanier L. L., Cwirla S., Phillips J. H. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989 Nov 15;143(10):3183–3191. [PubMed] [Google Scholar]
- Nagler A., Lanier L. L., Phillips J. H. Constitutive expression of high affinity interleukin 2 receptors on human CD16-natural killer cells in vivo. J Exp Med. 1990 May 1;171(5):1527–1533. doi: 10.1084/jem.171.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussia B., Starr S., Abraham S., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. I. Characterization of the lymphocyte subset reactive with B73.1. J Immunol. 1983 May;130(5):2133–2141. [PubMed] [Google Scholar]
- Timonen T., Reynolds C. W., Ortaldo J. R., Herberman R. B. Isolation of human and rat natural killer cells. J Immunol Methods. 1982;51(3):269–277. doi: 10.1016/0022-1759(82)90393-3. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kitamura F., Miyasaka M. Characterization of the interleukin 2 receptor beta chain using three distinct monoclonal antibodies. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1982–1986. doi: 10.1073/pnas.86.6.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren H. S., Atkinson K., Pembrey R. G., Biggs J. C. Human bone marrow allograft recipients: production of, and responsiveness to, interleukin 2. J Immunol. 1983 Oct;131(4):1771–1775. [PubMed] [Google Scholar]
- Warren H. S., Bezos A. Heterogeneity in the activation requirements of T cells stimulated by phytohaemagglutinin. Immunology. 1987 Jun;61(2):167–172. [PMC free article] [PubMed] [Google Scholar]
- Warren H. S. Differentiation of NK-like cells from OKT3-, OKT11+, and OKM1+ small resting lymphocytes by culture with autologous T cell blasts and lymphokine. J Immunol. 1984 Jun;132(6):2888–2894. [PubMed] [Google Scholar]
- Warren H. S., Pembrey R. G. A method for the production and quantitative assay of human lymphokine preparations. J Immunol Methods. 1981;41(1):9–21. doi: 10.1016/0022-1759(81)90269-6. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Pembrey R. G. Cyclosporin inhibits a two-signal mechanism for the generation of cytotoxic NK-like cells, from small lymphocyte precursors. Immunol Lett. 1986 Mar;12(2-3):69–75. doi: 10.1016/0165-2478(86)90085-4. [DOI] [PubMed] [Google Scholar]
- Warren H. S. Resting (IL-2 non-responsive) precursors of human T cell receptor gamma delta-positive cells (TCR1 cells) are activated by a two signal process. Immunol Cell Biol. 1989 Apr;67(Pt 2):121–126. doi: 10.1038/icb.1989.17. [DOI] [PubMed] [Google Scholar]


