Abstract
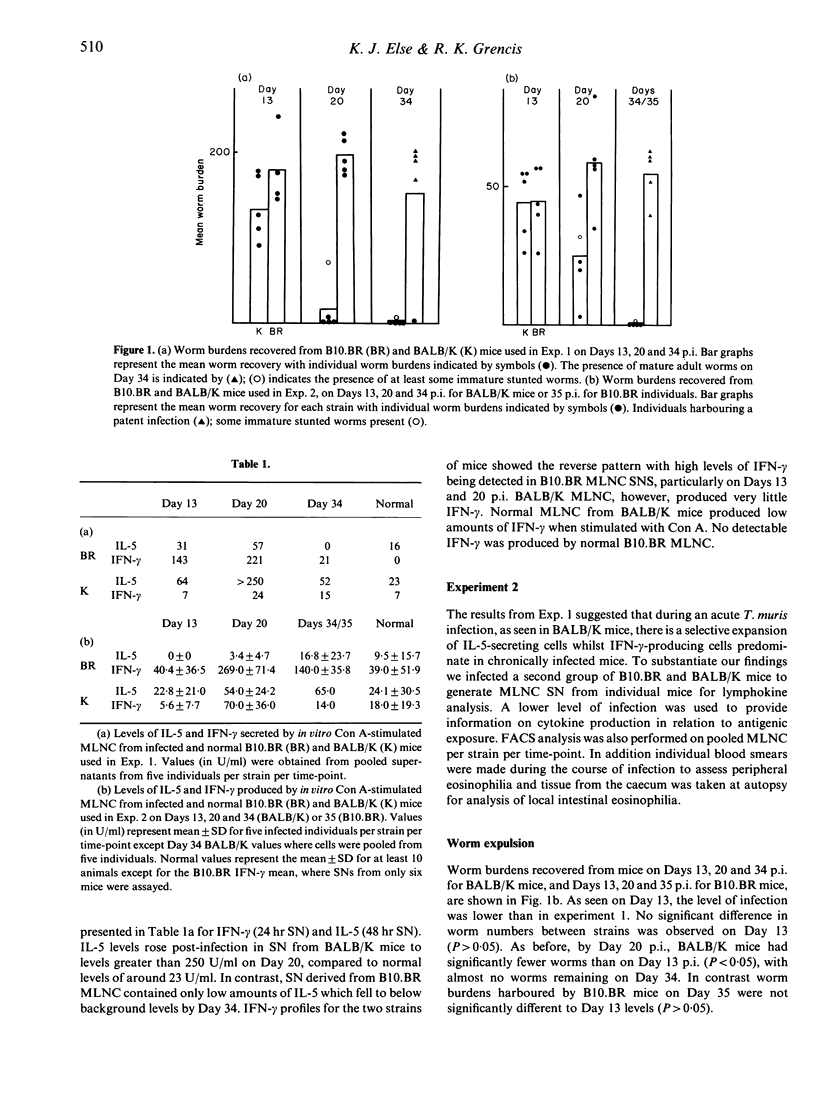
A variety of T-cell parameters have been analysed in two H-2 compatible strains of mice, B10.BR and BALB/K, which differ in an absolute fashion in their ability to resist infection with the parasitic nematode Trichuris muris: BALB/K mice expel T. muris relatively rapidly, whereas B10.BR mice are unable to expel the parasite before the infection reaches patency. Analysis of Th1- and Th2-specific cytokines (IFN-gamma and IL-5, respectively) produced by in vitro Con A-stimulated mesenteric lymph node cells (MLNC) from infected and normal mice demonstrated that MLNC from resistant BALB/K mice produced high levels of IL-5 and low levels of IFN-gamma whilst B10.BR MLNC secreted large amounts of IFN-gamma in the relative absence of IL-5. As an in vivo correlate of in vitro IL-5 production, peripheral and tissue eosinophilia were quantified during the course of infection in the two strains of mice. No peripheral eosinophilia was observed in BALB/K or B10.BR individuals. However, a considerable intestinal eosinophilia was seen in the high IL-5-producing BALB/K mice compared to normal levels. Differences observed in cytokine profiles were not due to differential changes in the numbers of T cells within the MLN. Indeed, FACS analysis revealed a decrease in the relative percentage of CD4+ and CD8+ T cells in both strains of mice post-infection. Our results suggest that resistance to T. muris involves the preferential induction of Th cells which secrete IL-5, whilst cells of a different Th subset (IFN-gamma producing) predominate in chronically infected mice. As such, this represents the first description of a correlation between the reciprocal activation of Th cell subsets in relation to acute or chronic intestinal infection with the same parasite in the same host species.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Shrader B., Carty J., Mosmann T. R., Bond M. W. A mouse T cell product that preferentially enhances IgA production. I. Biologic characterization. J Immunol. 1987 Dec 1;139(11):3685–3690. [PubMed] [Google Scholar]
- Else K. J., Wakelin D., Wassom D. L., Hauda K. M. MHC-restricted antibody responses to Trichuris muris excretory/secretory (E/S) antigen. Parasite Immunol. 1990 Sep;12(5):509–527. doi: 10.1111/j.1365-3024.1990.tb00985.x. [DOI] [PubMed] [Google Scholar]
- Else K. J., Wakelin D., Wassom D. L., Hauda K. M. The influence of genes mapping within the major histocompatibility complex on resistance to Trichuris muris infections in mice. Parasitology. 1990 Aug;101(Pt 1):61–67. doi: 10.1017/s0031182000079762. [DOI] [PubMed] [Google Scholar]
- Else K., Wakelin D. The effects of H-2 and non-H-2 genes on the expulsion of the nematode Trichuris muris from inbred and congenic mice. Parasitology. 1988 Jun;96(Pt 3):543–550. doi: 10.1017/s0031182000080173. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Schell S. R., Fitch F. W. Evidence implicating utilization of different T cell receptor-associated signaling pathways by TH1 and TH2 clones. J Immunol. 1990 Jun 1;144(11):4110–4120. [PubMed] [Google Scholar]
- Goodman T., Lefrancois L. Intraepithelial lymphocytes. Anatomical site, not T cell receptor form, dictates phenotype and function. J Exp Med. 1989 Nov 1;170(5):1569–1581. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Spitalny G. L. Production and characterization of anti-murine interferon-gamma sera. J Interferon Res. 1983;3(2):191–198. doi: 10.1089/jir.1983.3.191. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo R. T., Born W., Kappler J. W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989 Apr 15;142(8):2736–2742. [PubMed] [Google Scholar]
- Lee T. D., Wakelin D., Grencis R. K. Cellular mechanisms of immunity to the nematode Trichuris muris. Int J Parasitol. 1983 Aug;13(4):349–353. doi: 10.1016/s0020-7519(83)80039-3. [DOI] [PubMed] [Google Scholar]
- Lee T. D., Wakelin D. The use of host strain variation to assess the significance of mucosal mast cells in the spontaneous cure response of mice to the nematode Trichuris muris. Int Arch Allergy Appl Immunol. 1982;67(4):302–305. doi: 10.1159/000233037. [DOI] [PubMed] [Google Scholar]
- Limaye A. P., Abrams J. S., Silver J. E., Ottesen E. A., Nutman T. B. Regulation of parasite-induced eosinophilia: selectively increased interleukin 5 production in helminth-infected patients. J Exp Med. 1990 Jul 1;172(1):399–402. doi: 10.1084/jem.172.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Fong T. A. Specific assays for cytokine production by T cells. J Immunol Methods. 1989 Jan 17;116(2):151–158. doi: 10.1016/0022-1759(89)90198-1. [DOI] [PubMed] [Google Scholar]
- Murray P. D., McKenzie D. T., Swain S. L., Kagnoff M. F. Interleukin 5 and interleukin 4 produced by Peyer's patch T cells selectively enhance immunoglobulin A expression. J Immunol. 1987 Oct 15;139(8):2669–2674. [PubMed] [Google Scholar]
- Pond L., Wassom D. L., Hayes C. E. Evidence for differential induction of helper T cell subsets during Trichinella spiralis infection. J Immunol. 1989 Dec 15;143(12):4232–4237. [PubMed] [Google Scholar]
- Roach T. I., Else K. J., Wakelin D., McLaren D. J., Grencis R. K. Trichuris muris: antigen recognition and transfer of immunity in mice by IgA monoclonal antibodies. Parasite Immunol. 1991 Jan;13(1):1–12. doi: 10.1111/j.1365-3024.1991.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., Campbell H. D., Young I. G. Molecular and cellular biology of eosinophil differentiation factor (interleukin-5) and its effects on human and mouse B cells. Immunol Rev. 1988 Feb;102:29–50. doi: 10.1111/j.1600-065x.1988.tb00740.x. [DOI] [PubMed] [Google Scholar]
- Schmitt E., Van Brandwijk R., Fischer H. G., Rüde E. Establishment of different T cell sublines using either interleukin 2 or interleukin 4 as growth factors. Eur J Immunol. 1990 Aug;20(8):1709–1715. doi: 10.1002/eji.1830200813. [DOI] [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Wakelin D. Acquired immunity to Trichuris muris in the albino laboratory mouse. Parasitology. 1967 Aug;57(3):515–524. doi: 10.1017/s0031182000072395. [DOI] [PubMed] [Google Scholar]


