Abstract
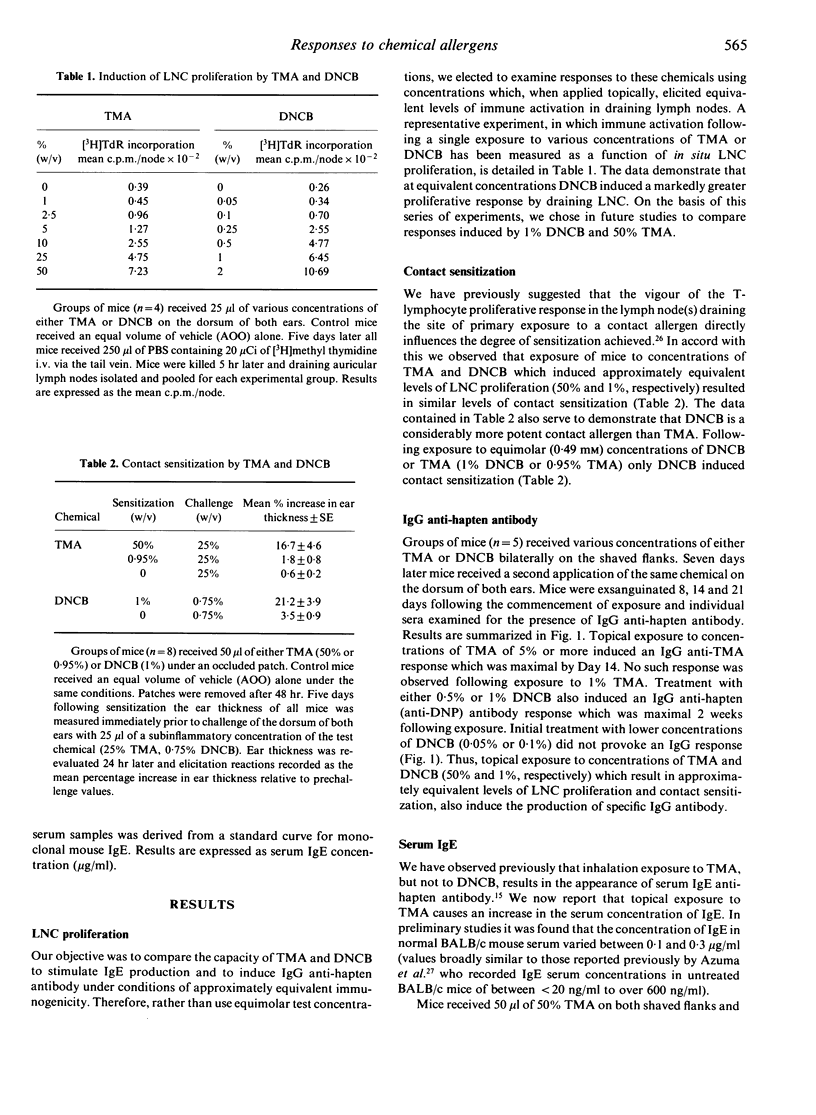
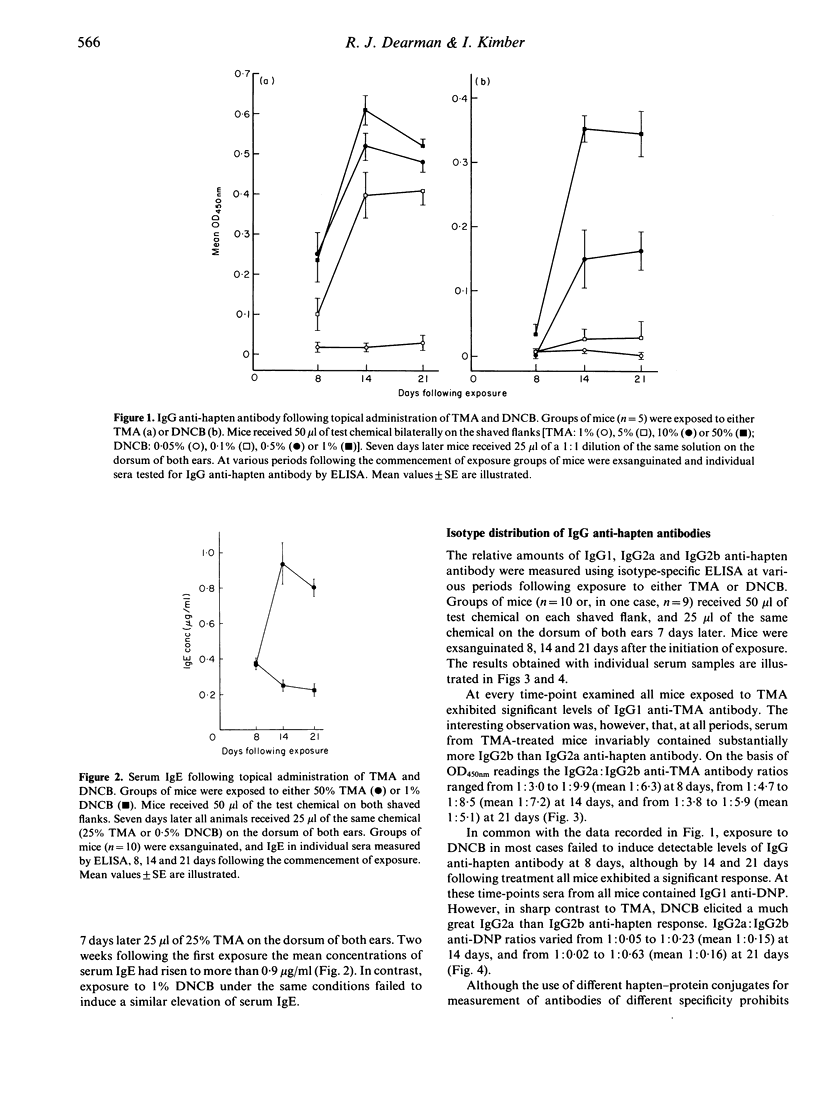
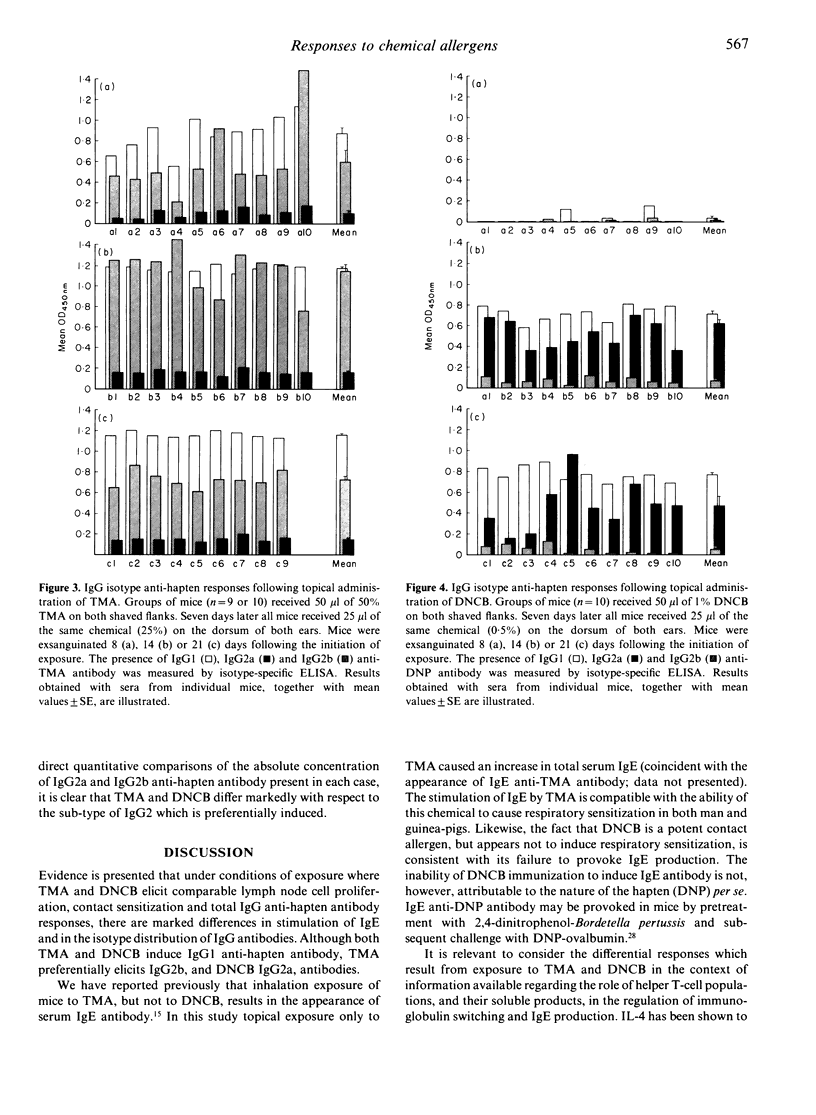
The nature of immune responses induced following topical exposure to 2,4-dinitrochlorobenzene (DNCB), a potent contact allergen which lacks the capacity to cause respiratory sensitization, and trimellitic anhydride (TMA), a respiratory allergen with comparatively weak skin-sensitizing potential, have been investigated. Exposure of BALB/c strain mice to concentrations of TMA and DNCB which resulted in equivalent levels of activation (cell proliferation) in lymph nodes draining the site of application (50% TMA and 1% DNCB) induced comparable levels of contact sensitization and IgG anti-hapten antibody production. However, under these conditions, exposure only to TMA resulted in an elevation of serum IgE. Furthermore, while TMA induced IgG2b rather than IgG2a antibody the reverse pattern was observed with DNCB. These data demonstrate that TMA and DNCB elicit qualitatively different immune responses which are consistent with their potential to cause respiratory and contact allergy, respectively. The possibility that the responses induced by these chemicals reflect a differential stimulation of T-helper cell subsets (Th1 and Th2) is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma M., Hirano T., Miyajima H., Watanabe N., Yagita H., Enomoto S., Furusawa S., Ovary Z., Kinashi T., Honjo T. Regulation of murine IgE production in SJA/9 and nude mice. Potentiation of IgE production by recombinant interleukin 4. J Immunol. 1987 Oct 15;139(8):2538–2544. [PubMed] [Google Scholar]
- Bass H., Mosmann T., Strober S. Evidence for mouse Th1- and Th2-like helper T cells in vivo. Selective reduction of Th1-like cells after total lymphoid irradiation. J Exp Med. 1989 Nov 1;170(5):1495–1511. doi: 10.1084/jem.170.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur X., Dewair M., Fruhmann G. Detection of immunologically sensitized isocyanate workers by RAST and intracutaneous skin tests. J Allergy Clin Immunol. 1984 May;73(5 Pt 1):610–618. doi: 10.1016/0091-6749(84)90520-7. [DOI] [PubMed] [Google Scholar]
- Bernstein D. I., Patterson R., Zeiss C. R. Clinical and immunologic evaluation of trimellitic anhydride-and phthalic anhydride-exposed workers using a questionnaire with comparative analysis of enzyme-linked immunosorbent and radioimmunoassay studies. J Allergy Clin Immunol. 1982 Mar;69(3):311–318. doi: 10.1016/s0091-6749(82)80009-2. [DOI] [PubMed] [Google Scholar]
- Boss W. F., Kelley C. J., Landsberger F. R. A novel synthesis of spin label derivatives of phosphatidylcholine. Anal Biochem. 1975 Mar;64(1):289–292. doi: 10.1016/0003-2697(75)90432-7. [DOI] [PubMed] [Google Scholar]
- Botham P. A., Hext P. M., Rattray N. J., Walsh S. T., Woodcock D. R. Sensitisation of guinea pigs by inhalation exposure to low molecular weight chemicals. Toxicol Lett. 1988 May;41(2):159–173. doi: 10.1016/0378-4274(88)90089-6. [DOI] [PubMed] [Google Scholar]
- Botham P. A., Rattray N. J., Woodcock D. R., Walsh S. T., Hext P. M. The induction of respiratory allergy in guinea-pigs following intradermal injection of trimellitic anhydride: a comparison with the response to 2,4-dinitrochlorobenzene. Toxicol Lett. 1989 Apr;47(1):25–39. doi: 10.1016/0378-4274(89)90083-0. [DOI] [PubMed] [Google Scholar]
- Butcher B. T., Salvaggio J. E. Occupational asthma. J Allergy Clin Immunol. 1986 Oct;78(4 Pt 1):547–556. doi: 10.1016/0091-6749(86)90068-0. [DOI] [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986 Feb 1;136(3):949–954. [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Coffman R. L., Seymour B. W., Lebman D. A., Hiraki D. D., Christiansen J. A., Shrader B., Cherwinski H. M., Savelkoul H. F., Finkelman F. D., Bond M. W. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988 Feb;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Danks J. M., Cromwell O., Buckingham J. A., Newman-Taylor A. J., Davies R. J. Toluene-diisocyanate induced asthma: evaluation of antibodies in the serum of affected workers against a tolyl mono-isocyanate protein conjugate. Clin Allergy. 1981 Mar;11(2):161–168. doi: 10.1111/j.1365-2222.1981.tb01580.x. [DOI] [PubMed] [Google Scholar]
- Diamantstein T., Eckert R., Volk H. D., Kupier-Weglinski J. W. Reversal by interferon-gamma of inhibition of delayed-type hypersensitivity induction by anti-CD4 or anti-interleukin 2 receptor (CD25) monoclonal antibodies. Evidence for the physiological role of the CD4+ TH1+ subset in mice. Eur J Immunol. 1988 Dec;18(12):2101–2103. doi: 10.1002/eji.1830181237. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Holmes J., Ohara J., Tung A. S., Sample J. V., Paul W. E. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988 Oct 1;141(7):2335–2341. [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Snapper C. M., Ohara J., Paul W. E. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9675–9678. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- Gilbert K. M., Hoang K. D., Weigle W. O. Th1 and Th2 clones differ in their response to a tolerogenic signal. J Immunol. 1990 Mar 15;144(6):2063–2071. [PubMed] [Google Scholar]
- Hagen M., Essani N. A., Strejan G. H. Role of interferon-gamma in the modulation of the IgE response by 2,4-dinitrophenyl-Bordetella pertussis vaccine in the mouse. Eur J Immunol. 1989 Mar;19(3):441–446. doi: 10.1002/eji.1830190305. [DOI] [PubMed] [Google Scholar]
- Howe W., Venables K. M., Topping M. D., Dally M. B., Hawkins R., Law J. S., Taylor A. J. Tetrachlorophthalic anhydride asthma: evidence for specific IgE antibody. J Allergy Clin Immunol. 1983 Jan;71(1 Pt 1):5–11. doi: 10.1016/0091-6749(83)90539-0. [DOI] [PubMed] [Google Scholar]
- Isakson P. C., Puré E., Vitetta E. S., Krammer P. H. T cell-derived B cell differentiation factor(s). Effect on the isotype switch of murine B cells. J Exp Med. 1982 Mar 1;155(3):734–748. doi: 10.1084/jem.155.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagouni E. E., Hadjipetrou-Kourounakis L. Regulation of isotype immunoglobulin production by adjuvants in vivo. Scand J Immunol. 1990 Jun;31(6):745–754. doi: 10.1111/j.1365-3083.1990.tb02826.x. [DOI] [PubMed] [Google Scholar]
- Karol M. H., Dixon C., Brady M., Alarie Y. Immunologic sensitization and pulmonary hypersensitivity by repeated inhalation of aromatic isocyanates. Toxicol Appl Pharmacol. 1980 Apr;53(2):260–270. doi: 10.1016/0041-008x(80)90425-1. [DOI] [PubMed] [Google Scholar]
- Karol M. H., Ioset H. H., Alarie Y. C. Tolyl-specific IgE antibodies in workders with hypersensitivity to toluene diisocyanate. Am Ind Hyg Assoc J. 1978 Jun;39(6):454–458. doi: 10.1080/0002889778507789. [DOI] [PubMed] [Google Scholar]
- Karol M. H., Ioset H. H., Riley E. J., Alarie Y. C. Hapten-specific respiratory hypersensitivity in guinea pigs. Am Ind Hyg Assoc J. 1978 Jul;39(7):546–556. doi: 10.1080/0002889778507807. [DOI] [PubMed] [Google Scholar]
- Karol M. H. The development of an animal model for TDI asthma. Bull Eur Physiopathol Respir. 1987 Nov-Dec;23(6):571–576. [PubMed] [Google Scholar]
- Kimber I., Shepherd C. J., Mitchell J. A., Turk J. L., Baker D. Regulation of lymphocyte proliferation in contact sensitivity: homeostatic mechanisms and a possible explanation of antigenic competition. Immunology. 1989 Apr;66(4):577–582. [PMC free article] [PubMed] [Google Scholar]
- McHeyzer-Williams M. G. Combinations of interleukins 2, 4 and 5 regulate the secretion of murine immunoglobulin isotypes. Eur J Immunol. 1989 Nov;19(11):2025–2030. doi: 10.1002/eji.1830191109. [DOI] [PubMed] [Google Scholar]
- Moller D. R., Gallagher J. S., Bernstein D. I., Wilcox T. G., Burroughs H. E., Bernstein I. L. Detection of IgE-mediated respiratory sensitization in workers exposed to hexahydrophthalic anhydride. J Allergy Clin Immunol. 1985 Jun;75(6):663–672. doi: 10.1016/0091-6749(85)90091-0. [DOI] [PubMed] [Google Scholar]
- Moore K. W., Vieira P., Fiorentino D. F., Trounstine M. L., Khan T. A., Mosmann T. R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990 Jun 8;248(4960):1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Murray J. S., Madri J., Tite J., Carding S. R., Bottomly K. MHC control of CD4+ T cell subset activation. J Exp Med. 1989 Dec 1;170(6):2135–2140. doi: 10.1084/jem.170.6.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandor M., Gajewski T., Thorson J., Kemp J. D., Fitch F. W., Lynch R. G. CD4+ murine T cell clones that express high levels of immunoglobulin binding belong to the interleukin 4-producing T helper cell type 2 subset. J Exp Med. 1990 Jun 1;171(6):2171–2176. doi: 10.1084/jem.171.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Rennick D. M. Characterization of a murine lymphokine distinct from interleukin 2 and interleukin 3 (IL-3) possessing a T-cell growth factor activity and a mast-cell growth factor activity that synergizes with IL-3. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1857–1861. doi: 10.1073/pnas.83.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., Finkelman F. D., Paul W. E. Regulation of IgG1 and IgE production by interleukin 4. Immunol Rev. 1988 Feb;102:51–75. doi: 10.1111/j.1600-065x.1988.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Stevens T. L., Bossie A., Sanders V. M., Fernandez-Botran R., Coffman R. L., Mosmann T. R., Vitetta E. S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988 Jul 21;334(6179):255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Ohara J., Myers C. D., Layton J. E., Krammer P. H., Paul W. E. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B cell differentiation factor for IgG1. J Exp Med. 1985 Nov 1;162(5):1726–1731. doi: 10.1084/jem.162.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C. T., Hawrylowicz C. M., Unanue E. R. T helper cell subsets require the expression of distinct costimulatory signals by antigen-presenting cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8181–8185. doi: 10.1073/pnas.85.21.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Coffman R. L., Hagiwara H., Rennick D. M., Takebe Y., Yokota K., Gemmell L., Shrader B., Yang G., Meyerson P. Isolation and characterization of lymphokine cDNA clones encoding mouse and human IgA-enhancing factor and eosinophil colony-stimulating factor activities: relationship to interleukin 5. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7388–7392. doi: 10.1073/pnas.84.21.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]


