Abstract
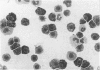
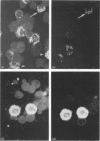
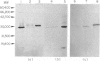
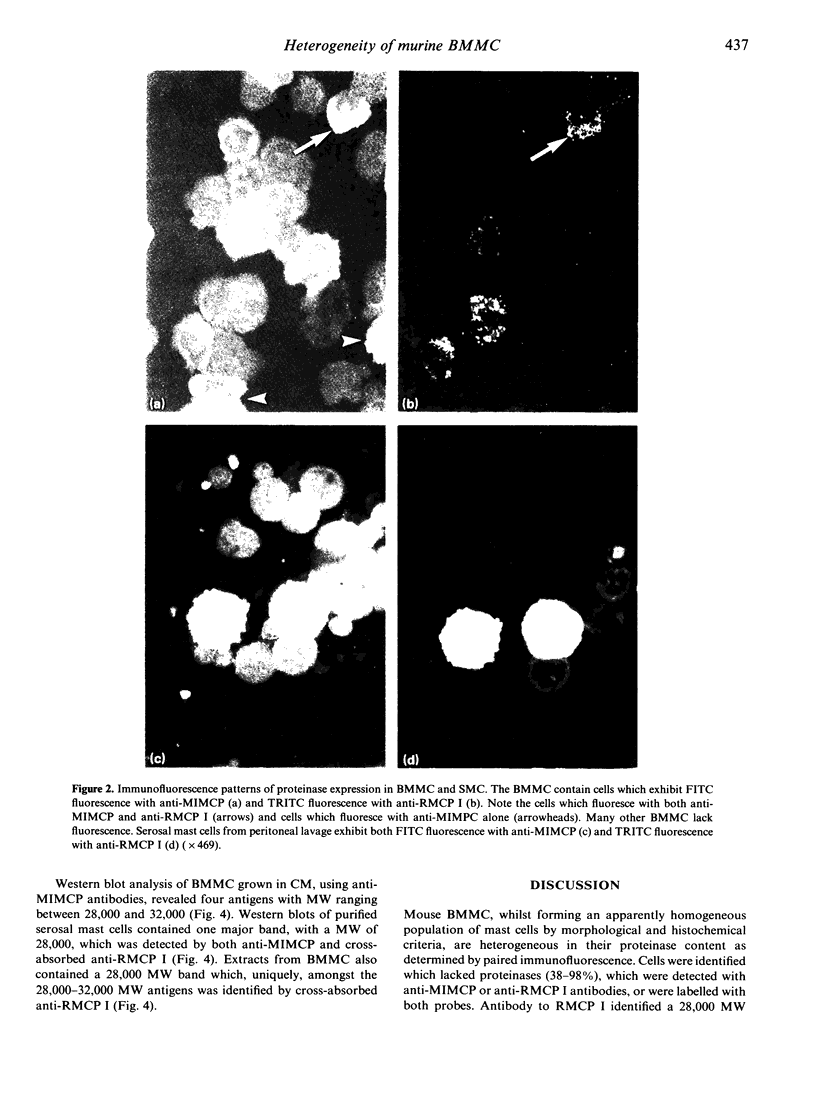
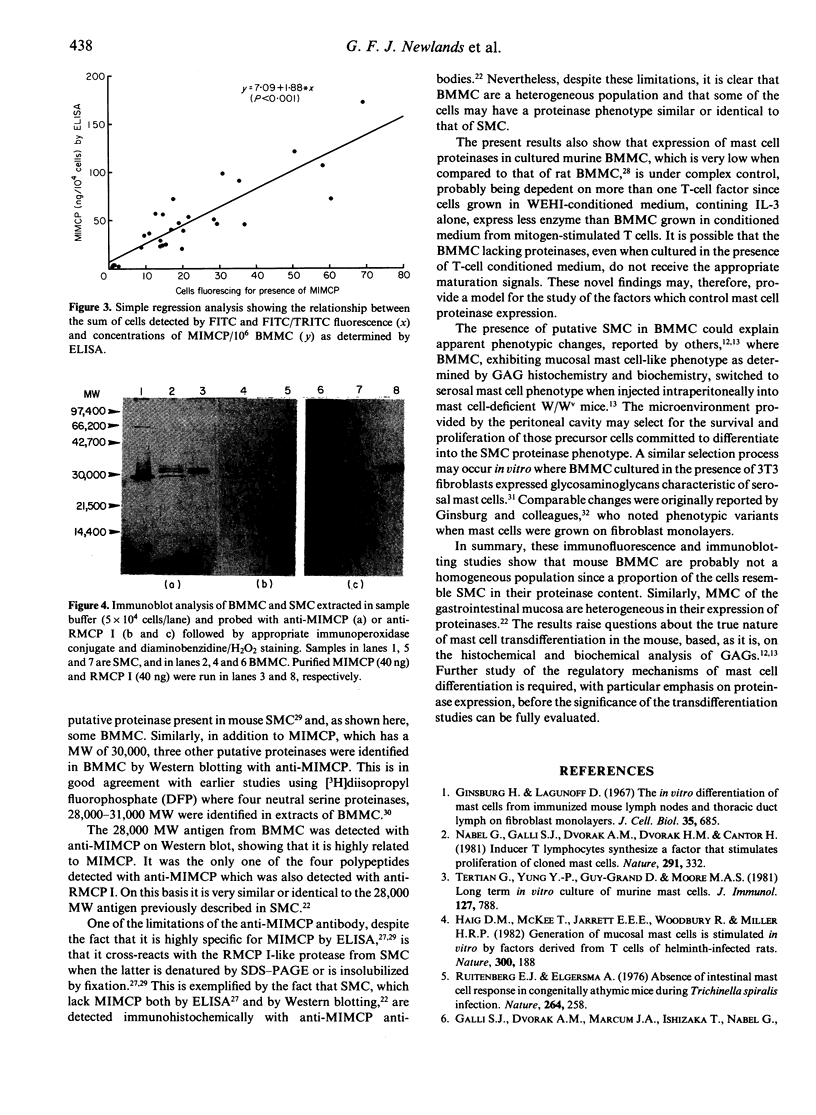
The expression of granule proteinases by murine bone marrow-derived mast cells (BMMC) grown in vitro was compared with that of serosal mast cells (SMC) from the peritoneal cavity. The granules in a proportion of BMMC (0.4-13%) and in all SMC were labelled with fluorescent antibodies against rat mast cell protease I (RMCP I). The granules of 1-47% of BMMC and 100% of SMC were labelled with antibodies against a 30,000 molecular weight (MW) murine intestinal mast cell proteinase (MIMCP). Four antigens from BMMC, ranging in MW from 28,000 to 32,000 and a single 28,000 antigen from SMC were detected on Western blot using anti-MIMCP antibodies. Only the 28,000 MW antigens from BMMC and SMC were visualized in blots probed with anti-RMCP I. BMMC grown in the presence of conditioned medium from activated splenocytes or from the WEHI-3B myelomonocytic cell line contained 52-118 ng and 3-25 ng MIMCP/10(6) cells respectively, whereas SMC lacked detectable MIMCP. The selective labelling of the 28,000 MW antigens in BMMC and SMC with anti-RMCP I and the variable expression of this antigen in BMMC as detected by immunofluorescence indicates that BMMC are not a homogeneous population of cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Befus A. D., Pearce F. L., Gauldie J., Horsewood P., Bienenstock J. Mucosal mast cells. I. Isolation and functional characteristics of rat intestinal mast cells. J Immunol. 1982 Jun;128(6):2475–2480. [PubMed] [Google Scholar]
- Combs J. W., Lagunoff D., Benditt E. P. Differentiation and proliferation of embryonic mast cells of the rat. J Cell Biol. 1965 Jun;25(3):577–592. doi: 10.1083/jcb.25.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle P. K., Phillips D. E. Characteristics of mast cells in Chediak-Higashi mice: light and electron microscopic studies of connective tissue and mucosal mast cells. Exp Cell Biol. 1983;51(3):130–139. doi: 10.1159/000163183. [DOI] [PubMed] [Google Scholar]
- DuBuske L., Austen K. F., Czop J., Stevens R. L. Granule-associated serine neutral proteases of the mouse bone marrow-derived mast cell that degrade fibronectin: their increase after sodium butyrate treatment of the cells. J Immunol. 1984 Sep;133(3):1535–1541. [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M., Marcum J. A., Ishizaka T., Nabel G., Der Simonian H., Pyne K., Goldin J. M., Rosenberg R. D., Cantor H. Mast cell clones: a model for the analysis of cellular maturation. J Cell Biol. 1982 Nov;95(2 Pt 1):435–444. doi: 10.1083/jcb.95.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S., Mackeller A., Newlands G. F., Miller H. R. Phenotypic expression of mast cell granule proteinases. Distribution of mast cell proteinases I and II in the rat digestive system. Immunology. 1987 Dec;62(4):621–627. [PMC free article] [PubMed] [Google Scholar]
- Gibson S., Miller H. R. Mast cell subsets in the rat distinguished immunohistochemically by their content of serine proteinases. Immunology. 1986 May;58(1):101–104. [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H., Ben-Shahar D., Ben-David E. Mast cell growth on fibroblast monolayers: two-cell entities. Immunology. 1982 Feb;45(2):371–380. [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H., Lagunoff D. The in vitro differentiation of mast cells. Cultures of cells from immunized mouse lymph nodes and thoracic duct lymph on fibroblast monolayers. J Cell Biol. 1967 Dec;35(3):685–697. doi: 10.1083/jcb.35.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D. M., McKee T. A., Jarrett E. E., Woodbury R., Miller H. R. Generation of mucosal mast cells is stimulated in vitro by factors derived from T cells of helminth-infected rats. Nature. 1982 Nov 11;300(5888):188–190. doi: 10.1038/300188a0. [DOI] [PubMed] [Google Scholar]
- Hamaguchi Y., Kanakura Y., Fujita J., Takeda S., Nakano T., Tarui S., Honjo T., Kitamura Y. Interleukin 4 as an essential factor for in vitro clonal growth of murine connective tissue-type mast cells. J Exp Med. 1987 Jan 1;165(1):268–273. doi: 10.1084/jem.165.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakura Y., Thompson H., Nakano T., Yamamura T., Asai H., Kitamura Y., Metcalfe D. D., Galli S. J. Multiple bidirectional alterations of phenotype and changes in proliferative potential during the in vitro and in vivo passage of clonal mast cell populations derived from mouse peritoneal mast cells. Blood. 1988 Sep;72(3):877–885. [PubMed] [Google Scholar]
- Katz H. R., Austen K. F., Caterson B., Stevens R. L. Secretory granules of heparin-containing rat serosal mast cells also possess highly sulfated chondroitin sulfate proteoglycans. J Biol Chem. 1986 Oct 15;261(29):13393–13396. [PubMed] [Google Scholar]
- Kitamura Y., Kanakura Y., Sonoda S., Asai H., Nakano T. Mutual phenotypic changes between connective tissue type and mucosal mast cells. Int Arch Allergy Appl Immunol. 1987;82(3-4):244–248. doi: 10.1159/000234198. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Hapel A. J., Ihle J. N. Constitutive production of a unique lymphokine (IL 3) by the WEHI-3 cell line. J Immunol. 1982 Jun;128(6):2393–2398. [PubMed] [Google Scholar]
- Levi-Schaffer F., Austen K. F., Gravallese P. M., Stevens R. L. Coculture of interleukin 3-dependent mouse mast cells with fibroblasts results in a phenotypic change of the mast cells. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6485–6488. doi: 10.1073/pnas.83.17.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin C. C., Gault E. A., Haig D. M. The effect of dexamethasone on growth and differentiation of bone-marrow derived mucosal mast cells in vitro. Immunology. 1987 Sep;62(1):29–34. [PMC free article] [PubMed] [Google Scholar]
- Miller H. R., Huntley J. F., Newlands G. F., Irvine J. Granule chymases and the characterization of mast cell phenotype and function in rat and mouse. Monogr Allergy. 1990;27:1–30. [PubMed] [Google Scholar]
- Miller H. R., Huntley J. F., Newlands G. F., Mackellar A., Lammas D. A., Wakelin D. Granule proteinases define mast cell heterogeneity in the serosa and the gastrointestinal mucosa of the mouse. Immunology. 1988 Dec;65(4):559–566. [PMC free article] [PubMed] [Google Scholar]
- Nabel G., Galli S. J., Dvorak A. M., Dvorak H. F., Cantor H. Inducer T lymphocytes synthesize a factor that stimulates proliferation of cloned mast cells. Nature. 1981 May 28;291(5813):332–334. doi: 10.1038/291332a0. [DOI] [PubMed] [Google Scholar]
- Nakano T., Sonoda T., Hayashi C., Yamatodani A., Kanayama Y., Yamamura T., Asai H., Yonezawa T., Kitamura Y., Galli S. J. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985 Sep 1;162(3):1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlands G. F., Gibson S., Knox D. P., Grencis R., Wakelin D., Miller H. R. Characterization and mast cell origin of a chymotrypsin-like proteinase isolated from intestines of mice infected with Trichinella spiralis. Immunology. 1987 Dec;62(4):629–634. [PMC free article] [PubMed] [Google Scholar]
- Otsu K., Nakano T., Kanakura Y., Asai H., Katz H. R., Austen K. F., Stevens R. L., Galli S. J., Kitamura Y. Phenotypic changes of bone marrow-derived mast cells after intraperitoneal transfer into W/Wv mice that are genetically deficient in mast cells. J Exp Med. 1987 Mar 1;165(3):615–627. doi: 10.1084/jem.165.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin E., Stevens R. L., Akiyama F., Schmid K., Austen K. F. Culture from mouse bone marrow of a subclass of mast cells possessing a distinct chondroitin sulfate proteoglycan with glycosaminoglycans rich in N-acetylgalactosamine-4,6-disulfate. J Biol Chem. 1982 Jun 25;257(12):7229–7236. [PubMed] [Google Scholar]
- Reynolds D. S., Stevens R. L., Lane W. S., Carr M. H., Austen K. F., Serafin W. E. Different mouse mast cell populations express various combinations of at least six distinct mast cell serine proteases. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3230–3234. doi: 10.1073/pnas.87.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg E. J., Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976 Nov 18;264(5583):258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- Serafin W. E., Katz H. R., Austen K. F., Stevens R. L. Complexes of heparin proteoglycans, chondroitin sulfate E proteoglycans, and [3H]diisopropyl fluorophosphate-binding proteins are exocytosed from activated mouse bone marrow-derived mast cells. J Biol Chem. 1986 Nov 15;261(32):15017–15021. [PubMed] [Google Scholar]
- Sredni B., Friedman M. M., Bland C. E., Metcalfe D. D. Ultrastructural, biochemical, and functional characteristics of histamine-containing cells cloned from mouse bone marrow: tentative identification as mucosal mast cells. J Immunol. 1983 Aug;131(2):915–922. [PubMed] [Google Scholar]
- Tertian G., Yung Y. P., Guy-Grand D., Moore M. A. Long-term in vitro culture of murine mast cells. I. Description of a growth factor-dependent culture technique. J Immunol. 1981 Aug;127(2):788–794. [PubMed] [Google Scholar]
- Woodbury R. G., Neurath H. Structure, specificity and localization of the serine proteases of connective tissue. FEBS Lett. 1980 Jun 2;114(2):189–196. doi: 10.1016/0014-5793(80)81112-4. [DOI] [PubMed] [Google Scholar]