Abstract
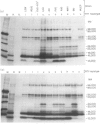
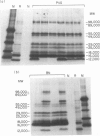
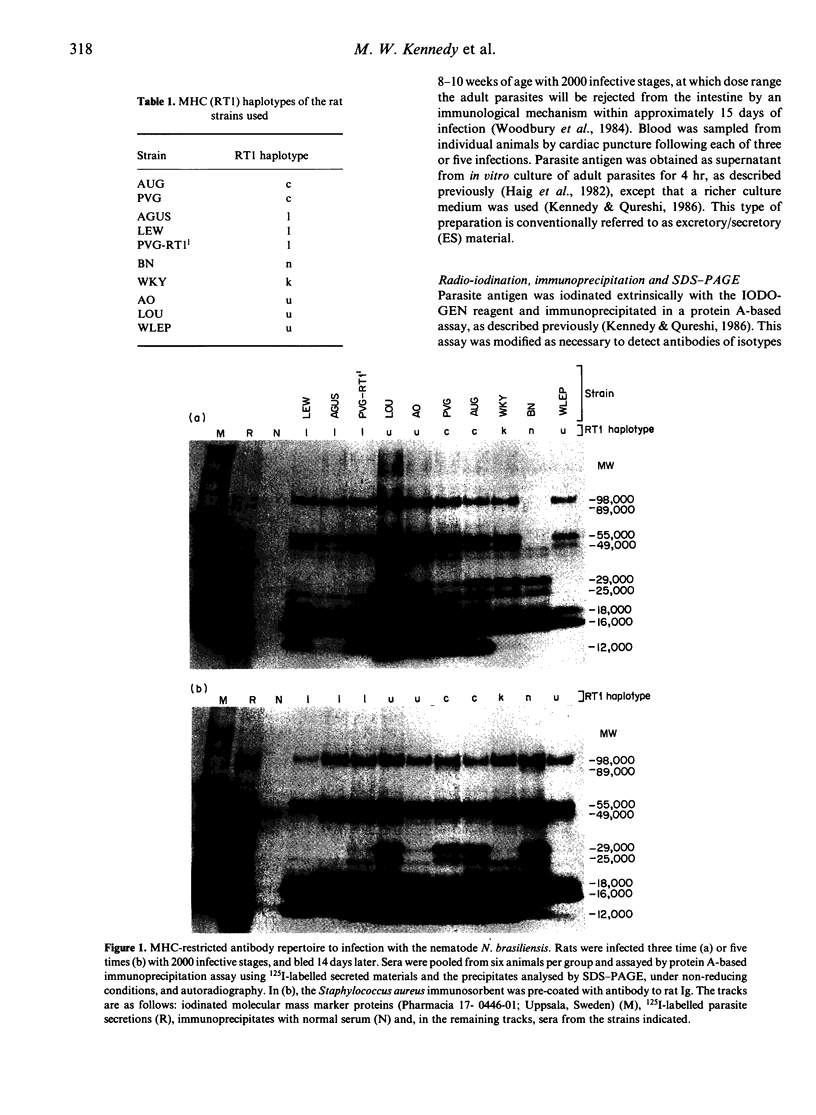
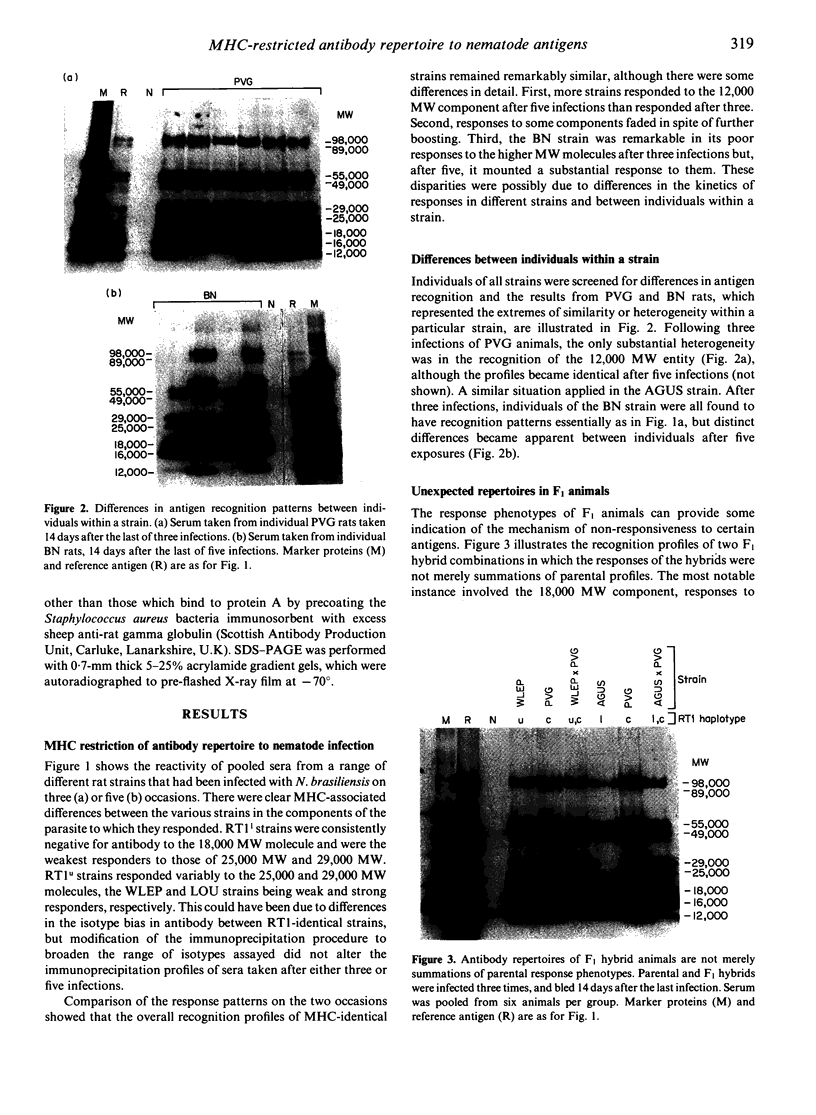
It might be expected that infections with transmissible agents will elicit an immune response to all of their exoantigens and that immune response (Ir) gene control of responses to individual epitopes on a given parasite component would be obscured by reaction to the molecule as a whole. Humans infected with parasitic nematodes, however, mount antibody responses which are selective for certain parasite components. This was modelled in inbred rats infected with the parasitic nematode Nippostrongylus brasiliensis and their responses to secreted antigens analysed by immunoprecipitation and SDS-PAGE. No strain responded to all the potential antigens and only those of identical major histocompatibility complex (MHC) had similar recognition profiles. This MHC-restricted response applied to whole molecules synthesized by the parasite, rather than merely to epitopes thereon and is, therefore, contrary to expectation. Moreover, the response patterns of F1 hybrid animals were not merely summations of parental responses. This suggests defective antigen presentation of particular parasite components by certain MHC class II molecules and/or cross-tolerance with background gene products.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F. Multiple cross-reactivities amongst antigens of Plasmodium falciparum impair the development of protective immunity against malaria. Parasite Immunol. 1986 Nov;8(6):529–539. doi: 10.1111/j.1365-3024.1986.tb00867.x. [DOI] [PubMed] [Google Scholar]
- Anderson R. M. The population dynamics and epidemiology of intestinal nematode infections. Trans R Soc Trop Med Hyg. 1986;80(5):686–696. doi: 10.1016/0035-9203(86)90367-6. [DOI] [PubMed] [Google Scholar]
- Barnett B. C., Graham C. M., Burt D. S., Skehel J. J., Thomas D. B. The immune response of BALB/c mice to influenza hemagglutinin: commonality of the B cell and T cell repertoires and their relevance to antigenic drift. Eur J Immunol. 1989 Mar;19(3):515–521. doi: 10.1002/eji.1830190316. [DOI] [PubMed] [Google Scholar]
- Bundy D. A. Population ecology of intestinal helminth infections in human communities. Philos Trans R Soc Lond B Biol Sci. 1988 Oct 31;321(1207):405–420. doi: 10.1098/rstb.1988.0100. [DOI] [PubMed] [Google Scholar]
- Christie J. F., Dunbar B., Davidson I., Kennedy M. W. N-terminal amino acid sequence identity between a major allergen of Ascaris lumbricoides and Ascaris suum, and MHC-restricted IgE responses to it. Immunology. 1990 Apr;69(4):596–602. [PMC free article] [PubMed] [Google Scholar]
- Del Giudice G., Cheng Q., Mazier D., Berbiguier N., Cooper J. A., Engers H. D., Chizzolini C., Verdini A. S., Bonelli F., Pessi A. Immunogenicity of a non-repetitive sequence of Plasmodium falciparum circumsporozoite protein in man and mice. Immunology. 1988 Feb;63(2):187–191. [PMC free article] [PubMed] [Google Scholar]
- Else K., Wakelin D. Genetic variation in the humoral immune responses of mice to the nematode Trichuris muris. Parasite Immunol. 1989 Jan;11(1):77–90. doi: 10.1111/j.1365-3024.1989.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Ir gene control of the murine secretory IgA response to cholera toxin. Eur J Immunol. 1987 Mar;17(3):425–428. doi: 10.1002/eji.1830170320. [DOI] [PubMed] [Google Scholar]
- Good M. F., Maloy W. L., Lunde M. N., Margalit H., Cornette J. L., Smith G. L., Moss B., Miller L. H., Berzofsky J. A. Construction of synthetic immunogen: use of new T-helper epitope on malaria circumsporozoite protein. Science. 1987 Feb 27;235(4792):1059–1062. doi: 10.1126/science.2434994. [DOI] [PubMed] [Google Scholar]
- Graham C. M., Barnett B. C., Hartlmayr I., Burt D. S., Faulkes R., Skehel J. J., Thomas D. B. The structural requirements for class II (I-Ad)-restricted T cell recognition of influenza hemagglutinin: B cell epitopes define T cell epitopes. Eur J Immunol. 1989 Mar;19(3):523–528. doi: 10.1002/eji.1830190317. [DOI] [PubMed] [Google Scholar]
- Haig D. M., McKee T. A., Jarrett E. E., Woodbury R., Miller H. R. Generation of mucosal mast cells is stimulated in vitro by factors derived from T cells of helminth-infected rats. Nature. 1982 Nov 11;300(5888):188–190. doi: 10.1038/300188a0. [DOI] [PubMed] [Google Scholar]
- Haig D. M., McMenamin C., Gunneberg C., Woodbury R., Jarrett E. E. Stimulation of mucosal mast cell growth in normal and nude rat bone marrow cultures. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4499–4503. doi: 10.1073/pnas.80.14.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. P., Bäckström B. T., Booth R. J., Love S. G., Harding D. R., Watson J. D. The mapping of epitopes of the 18-kDa protein of Mycobacterium leprae recognized by murine T cells in a proliferation assay. J Immunol. 1989 Sep 15;143(6):2006–2012. [PubMed] [Google Scholar]
- Infante A. J., Atassi M. Z., Fathman C. G. T cell clones reactive with sperm whale myoglobin. Isolation of clones with specificity for individual determinants on myoglobin. J Exp Med. 1981 Nov 1;154(5):1342–1356. doi: 10.1084/jem.154.5.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanyi J., Sharp K. Control by H-2 genes of murine antibody responses to protein antigens of Mycobacterium tuberculosis. Immunology. 1986 Nov;59(3):329–332. [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E., Miller H. R. Production and activities of IgE in helminth infection. Prog Allergy. 1982;31:178–233. [PubMed] [Google Scholar]
- Keck K. Ir-gene control of immunogenicity of insulin and A-chain loop as a carrier determinant. Nature. 1975 Mar 6;254(5495):78–79. doi: 10.1038/254078a0. [DOI] [PubMed] [Google Scholar]
- Kennedy M. W., Gordon A. M., Tomlinson L. A., Qureshi F. Genetic (major histocompatibility complex?) control of the antibody repertoire to the secreted antigens of Ascaris. Parasite Immunol. 1987 Mar;9(2):269–273. doi: 10.1111/j.1365-3024.1987.tb00506.x. [DOI] [PubMed] [Google Scholar]
- Kennedy M. W., Qureshi F. Stage-specific secreted antigens of the parasitic larval stages of the nematode Ascaris. Immunology. 1986 Jul;58(3):515–522. [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. W., Tomlinson L. A., Fraser E. M., Christie J. F. The specificity of the antibody response to internal antigens of Ascaris: heterogeneity in infected humans, and MHC (H-2) control of the repertoire in mice. Clin Exp Immunol. 1990 May;80(2):219–224. doi: 10.1111/j.1365-2249.1990.tb05237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Rees A. D., Bal V., Ikeda H., Wilkinson D., De Vries R. R., Rothbard J. B. Prediction and identification of an HLA-DR-restricted T cell determinant in the 19-kDa protein of Mycobacterium tuberculosis. Eur J Immunol. 1988 Jun;18(6):973–976. doi: 10.1002/eji.1830180623. [DOI] [PubMed] [Google Scholar]
- Matzinger P. A one-receptor view of T-cell behaviour. Nature. 1981 Aug 6;292(5823):497–501. doi: 10.1038/292497a0. [DOI] [PubMed] [Google Scholar]
- McLaren A., Tait A. Cytoplasmic isocitrate dehydrogenase variation within the C3H inbred strain. Genet Res. 1969 Aug;14(1):93–94. doi: 10.1017/s0016672300001890. [DOI] [PubMed] [Google Scholar]
- Miller H. R. The protective mucosal response against gastrointestinal nematodes in ruminants and laboratory animals. Vet Immunol Immunopathol. 1984 May;6(1-2):167–259. doi: 10.1016/0165-2427(84)90051-5. [DOI] [PubMed] [Google Scholar]
- Niiyama T., Kojima H., Mizuno K., Matsuno Y., Fujii H., Misonou J., Natori T., Aizawa M., Oikawa K. Genetic control of the immune responsiveness to Streptococcus mutans by the major histocompatibility complex of the rat (RT1). Infect Immun. 1987 Dec;55(12):3137–3141. doi: 10.1128/iai.55.12.3137-3141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit A., Pery P., Luffau G. Purification of an allergen from culture fluids of Nippostrongylus brasiliensis. Mol Immunol. 1980 Nov;17(11):1341–1349. doi: 10.1016/0161-5890(80)90003-6. [DOI] [PubMed] [Google Scholar]
- Rittner C., Schneider P. M. Complexity of MHC class III genes and complement polymorphism. Immunol Today. 1989 Dec;10(12):401–403. doi: 10.1016/0167-5699(89)90034-0. [DOI] [PubMed] [Google Scholar]
- Rosenwasser L. J., Barcinski M. A., Schwartz R. H., Rosenthal A. S. Immune response gene control of determinant selection. II. Genetic control of the murine T lymphocyte proliferative response to insulin. J Immunol. 1979 Jul;123(1):471–476. [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Schwartz R. Induction of tolerance to self in T lymphocytes. Nature. 1984 Apr 19;308(5961):690–691. doi: 10.1038/308690a0. [DOI] [PubMed] [Google Scholar]
- Stromberg B. E. Potentiation of the reaginic (IgE) antibody response to ovalbumin in the guinea pig with a soluble metabolic product from Ascaris suum. J Immunol. 1980 Aug;125(2):833–836. [PubMed] [Google Scholar]
- Togna A. R., Del Giudice G., Verdini A. S., Bonelli F., Pessi A., Engers H. D., Corradin G. Synthetic Plasmodium falciparum circumsporozoite peptides elicit heterogenous L3T4+ T cell proliferative responses in H-2b mice. J Immunol. 1986 Nov 1;137(9):2956–2960. [PubMed] [Google Scholar]
- Tomlinson L. A., Christie J. F., Fraser E. M., McLaughlin D., McIntosh A. E., Kennedy M. W. MHC restriction of the antibody repertoire to secretory antigens, and a major allergen, of the nematode parasite Ascaris. J Immunol. 1989 Oct 1;143(7):2349–2356. [PubMed] [Google Scholar]
- Vidović D., Matzinger P. Unresponsiveness to a foreign antigen can be caused by self-tolerance. Nature. 1988 Nov 17;336(6196):222–225. doi: 10.1038/336222a0. [DOI] [PubMed] [Google Scholar]
- Wassom D. L., Wakelin D., Brooks B. O., Krco C. J., David C. S. Genetic control of immunity to Trichinella spiralis infections of mice. Hypothesis to explain the role of H-2 genes in primary and challenge infections. Immunology. 1984 Apr;51(4):625–631. [PMC free article] [PubMed] [Google Scholar]
- Woodbury R. G., Miller H. R., Huntley J. F., Newlands G. F., Palliser A. C., Wakelin D. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infections in rat. 1984 Nov 29-Dec 5Nature. 312(5993):450–452. doi: 10.1038/312450a0. [DOI] [PubMed] [Google Scholar]