Abstract
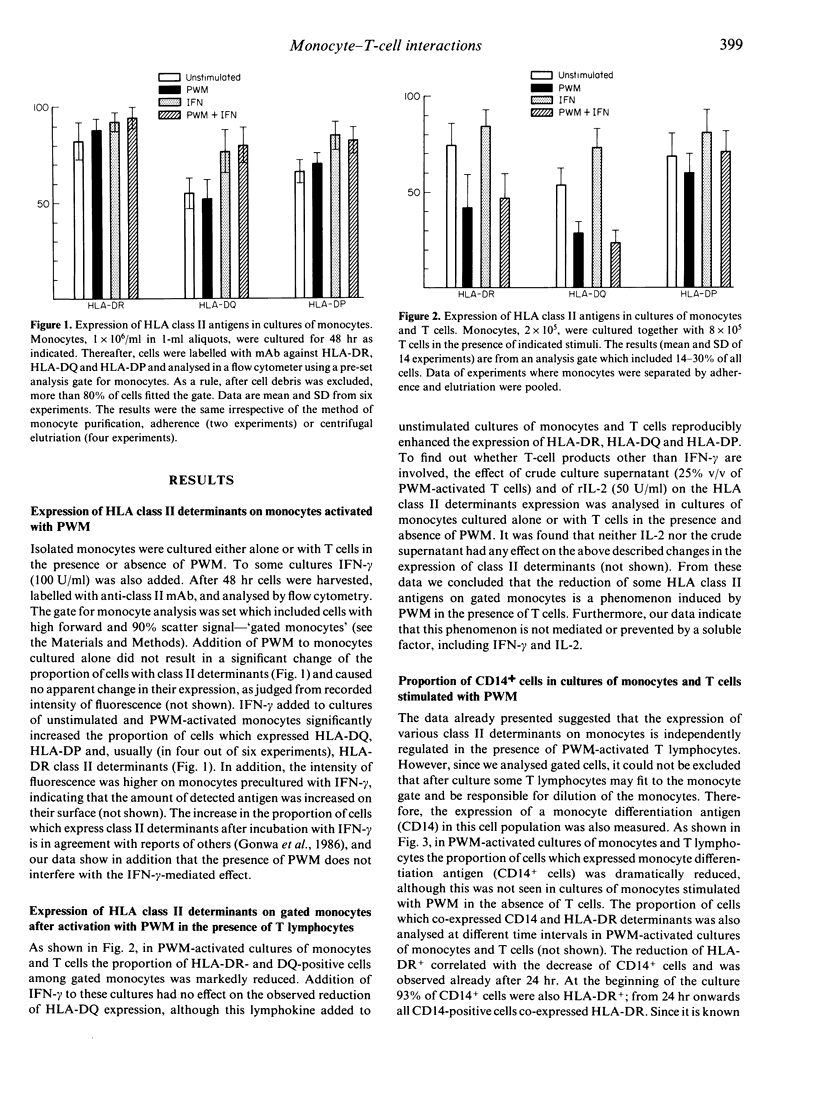
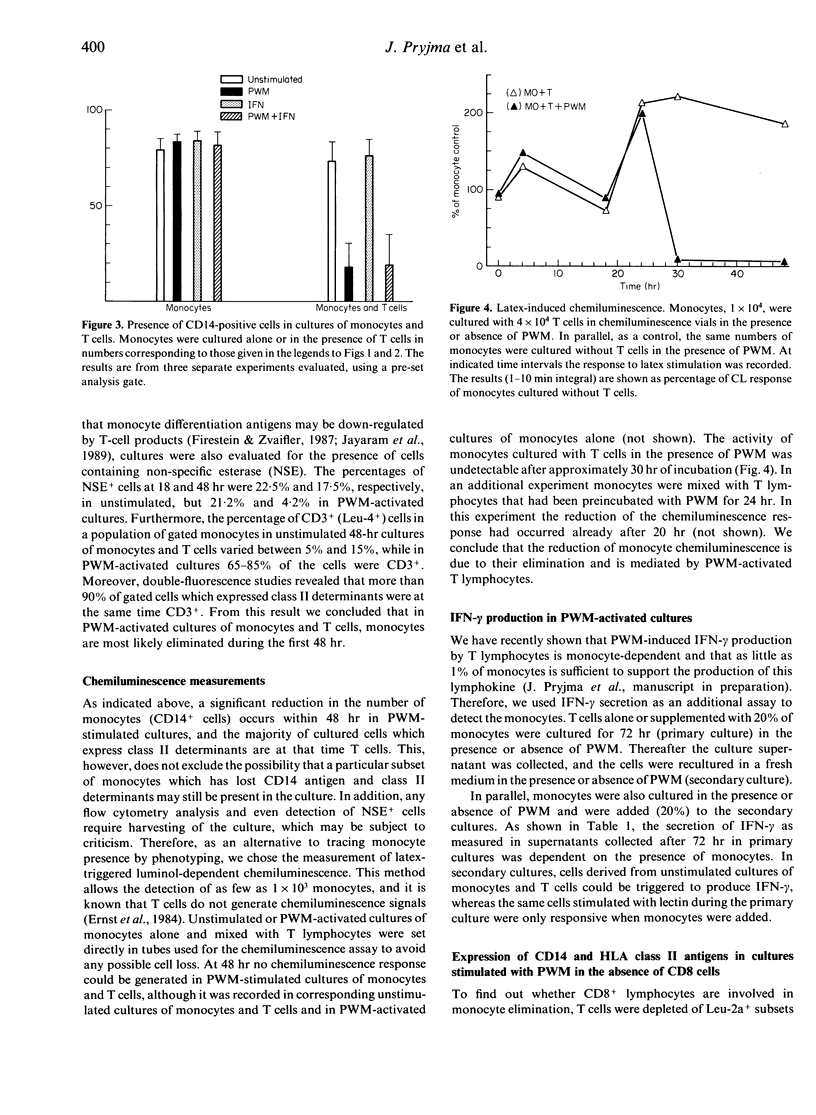
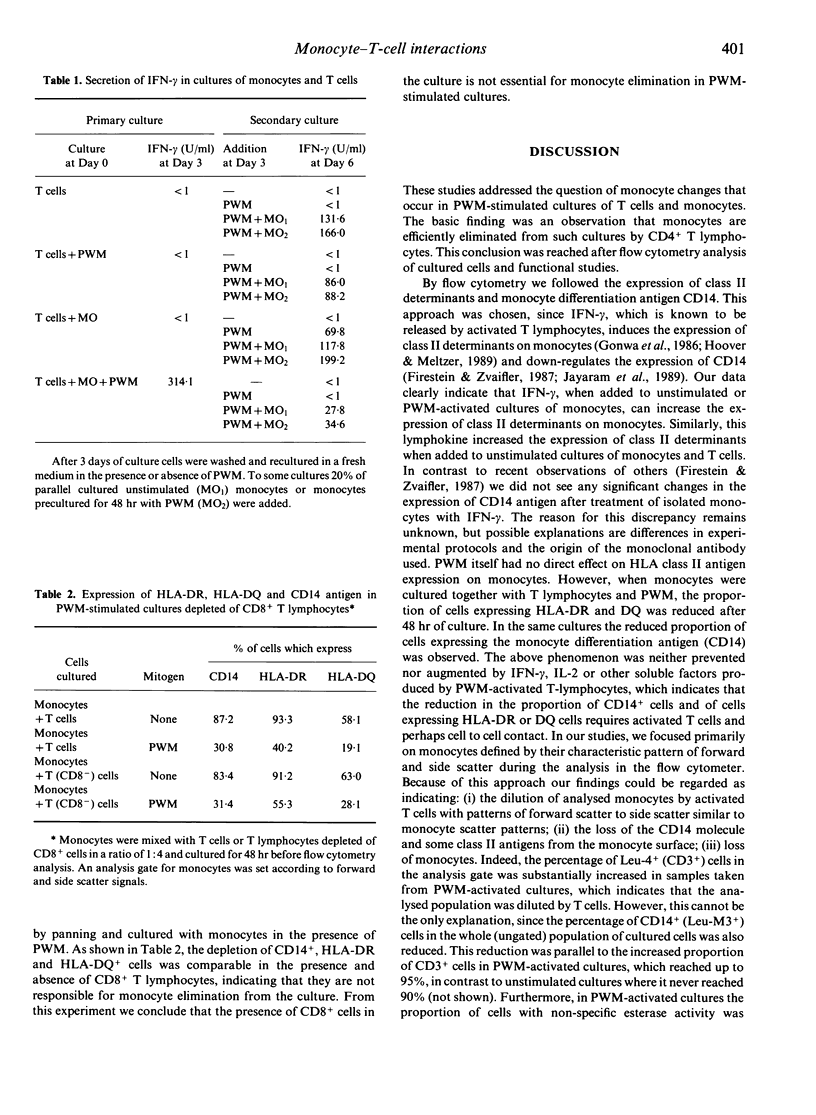
Monocyte-T-cell interactions were studied in pokeweed mitogen (PWM)-activated cell cultures. We addressed the question of monocyte changes in PWM-stimulated cultures of T cells and monocytes and found, by flow cytometric analysis, that PWM activation led to a loss of cells with monocyte or macrophage phenotype (CD14, HLA-DR, HLA-DQ) within 48 hr of culture in the presence of T cells (CD4+ T cells), but not in cultures of pure monocytes. Chemiluminescence measurements revealed that phagocytic stimulation of monocytic superoxide release was impaired in PWM-stimulated cultures of monocytes plus T cells, but not in PWM-stimulated cultures of pure monocytes. Furthermore, PWM induced the secretion of interferon-gamma (IFN-gamma) in primary cultures of T cells supplemented with 20% of monocytes, whereas in subsequent secondary cultures of these cells PWM induced IFN-gamma only when monocytes were added. We conclude from these flow cytometric and functional analyses that monocytes are efficiently eliminated from PWM-activated T-cell/monocyte cultures by CD4+ T lymphocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanchard D. K., Michelini-Norris M. B., Friedman H., Djeu J. Y. Lysis of mycobacteria-infected monocytes by IL-2-activated killer cells: role of LFA-1. Cell Immunol. 1989 Apr 1;119(2):402–411. doi: 10.1016/0008-8749(89)90254-2. [DOI] [PubMed] [Google Scholar]
- Diedrichs M., Schendel D. J. Differential surface expression of class II isotypes on activated CD4 and CD8 cells correlates with levels of locus-specific mRNA. J Immunol. 1989 May 1;142(9):3275–3280. [PubMed] [Google Scholar]
- Elmasry M. N., Fox E. J., Rich R. R. Opposing immunoregulatory functions of CD8+ lymphocytes: a requirement for monocytes in suppressor cell induction. J Immunol. 1986 Oct 15;137(8):2468–2477. [PubMed] [Google Scholar]
- Ernst M., Lange A., Flad H. D., Havel A., Ennen J., Ulmer A. J. Dissociation of responses measured by natural cytotoxicity and chemiluminescence. Eur J Immunol. 1984 Jul;14(7):634–639. doi: 10.1002/eji.1830140710. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. Down regulation of human monocyte differentiation antigens by interferon gamma. Cell Immunol. 1987 Feb;104(2):343–354. doi: 10.1016/0008-8749(87)90036-0. [DOI] [PubMed] [Google Scholar]
- Gansbacher B., Zier K. S. Regulation of HLA-DR, DP, and DQ expression in activated T cells. Cell Immunol. 1988 Nov;117(1):22–34. doi: 10.1016/0008-8749(88)90073-1. [DOI] [PubMed] [Google Scholar]
- Gonwa T. A., Frost J. P., Karr R. W. All human monocytes have the capability of expressing HLA-DQ and HLA-DP molecules upon stimulation with interferon-gamma. J Immunol. 1986 Jul 15;137(2):519–524. [PubMed] [Google Scholar]
- Hansen P. W., Petersen C. M., Povlsen J. V., Kristensen T. Cytotoxic human HLA class II restricted purified protein derivative-reactive T-lymphocyte clones. IV. Analysis of HLA restriction pattern and mycobacterial antigen specificity. Scand J Immunol. 1987 Mar;25(3):295–303. doi: 10.1111/j.1365-3083.1987.tb01075.x. [DOI] [PubMed] [Google Scholar]
- Jayaram Y., Buckle A. M., Hogg N. The Fc receptor, FcRI, and other activation molecules on human mononuclear phagocytes after treatment with interferon-gamma. Clin Exp Immunol. 1989 Mar;75(3):414–420. [PMC free article] [PubMed] [Google Scholar]
- Nieda M., Juji T., Imao S., Minami M. A role of HLA-DQ molecules of stimulator-adherent cells in the regulation of human autologous mixed lymphocyte reaction. J Immunol. 1988 Nov 1;141(9):2975–2979. [PubMed] [Google Scholar]
- Ottenhoff T. H., Ab B. K., Van Embden J. D., Thole J. E., Kiessling R. The recombinant 65-kD heat shock protein of Mycobacterium bovis Bacillus Calmette-Guerin/M. tuberculosis is a target molecule for CD4+ cytotoxic T lymphocytes that lyse human monocytes. J Exp Med. 1988 Nov 1;168(5):1947–1952. doi: 10.1084/jem.168.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryjma J., Flad H. D., Gruber M., Ernst M. Monocyte-T-cell interactions in the regulation of polyclonal B-cell response. Scand J Immunol. 1986 Jul;24(1):21–28. doi: 10.1111/j.1365-3083.1986.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Robbins P. A., Maino V. C., Warner N. L., Brodsky F. M. Activated T cells and monocytes have characteristic patterns of class II antigen expression. J Immunol. 1988 Aug 15;141(4):1281–1287. [PubMed] [Google Scholar]
- Thiele D. L., Kurosaka M., Lipsky P. E. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J Immunol. 1983 Nov;131(5):2282–2290. [PubMed] [Google Scholar]
- Waldmann T. A., Broder S. Polyclonal B-cell activators in the study of the regulation of immunoglobulin synthesis in the human system. Adv Immunol. 1982;32:1–63. doi: 10.1016/s0065-2776(08)60720-8. [DOI] [PubMed] [Google Scholar]
- Zembala M., Uracz W., Ruggiero I., Mytar B., Pryjma J. Isolation and functional characteristics of FcR+ and FcR- human monocyte subsets. J Immunol. 1984 Sep;133(3):1293–1299. [PubMed] [Google Scholar]


