Abstract
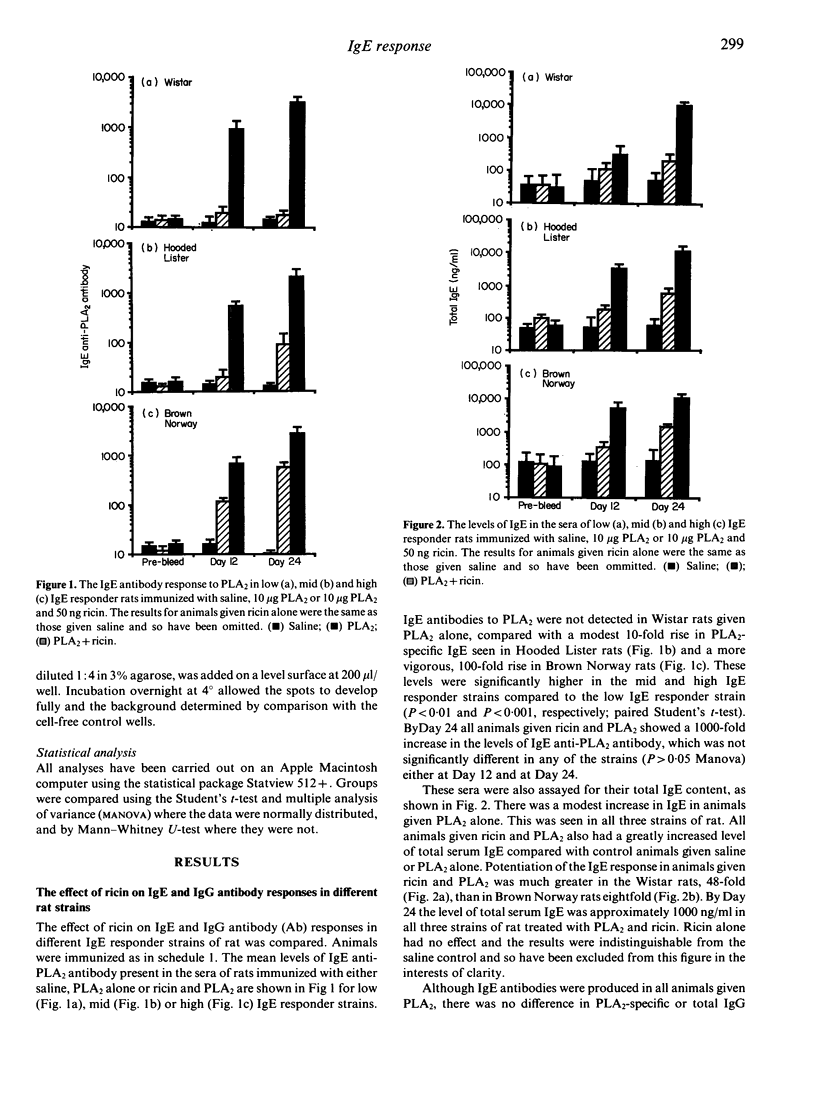
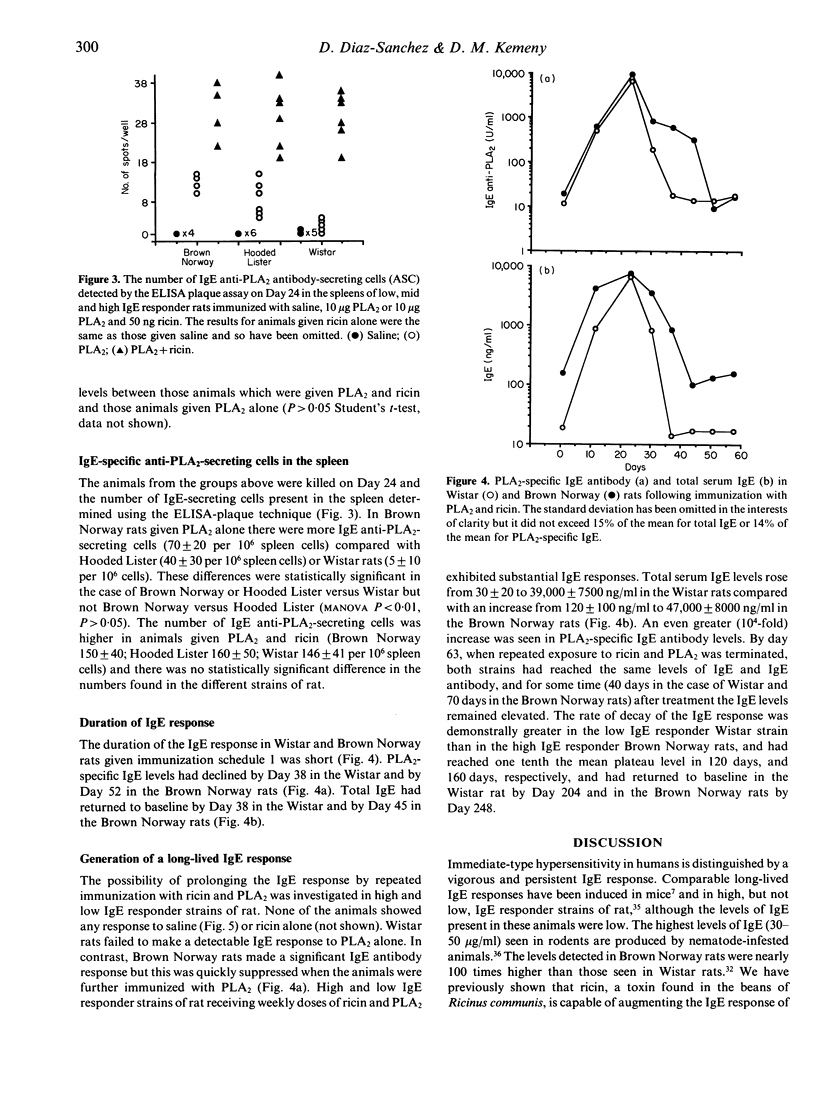
Certain strains of rats infested with the nematode parasite Nippostrongulus brasiliensis mount vigorous, persistent immunoglobulin E (IgE) responses. In the absence of parasites, adjuvants such as Bordatella pertussis or Al(OH)3 are needed to produce IgE responses to soluble antigens. These are short-lived, even in high IgE responder strains. In this study we have produced long-lived IgE responses in both low (Wistar) and high (Brown Norway) IgE responder strains of rats by repeated injections of ricin, a toxic lectin from castor beans, and phospholipase A2 (PLA2), a bee venom protein. Total IgE levels rose from 30 +/- 20 ng/ml to 39,000 +/- 7500 ng/ml in the Wistar rats compared with an increase from 120 +/- 100 ng/ml to 47,000 +/- 8000 ng/ml in the Brown Norway rats. An even greater (10(4)-fold) increase was seen in PLA2-specific IgE antibody levels. total and PLA2-specific IgE started to fall 6 weeks after treatment was stopped in the Wistar and after 12 weeks in the Brown Norway rats. The duration of the response was 204 and 248 days, respectively. The IgE-enhancing properties of ricin were compared in low, mid (Hooded Lister) and high IgE responder rats. Total IgE and PLA2-specific IgE but not IgG antibody (Ab) responses were enhanced in all animals given ricin and PLA2 but not in animals given ricin or PLA2 alone. The increase was greater in Wistar rats (48-fold) than in Brown Norway rats (eightfold) and by Day 24 the levels of both total and PLA2-specific IgE in three different strains were indistinguishable. PLA2-specific IgE antibody-secreting cells were detected in the spleen at a frequency of 1:5000. These results show: (i) that repeated immunization of rats with antigen and ricin produce a very large IgE response which was long-lived; (ii) that this response was indistinguishable in different IgE responder strains of rat; and (iii) that the IgE response declines earlier in low IgE responder strains of rats.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auchincloss H., Jr, Winn H. J. Murine CD8+ T cell helper function is particularly sensitive to cyclosporine suppression in vivo. J Immunol. 1989 Dec 15;143(12):3940–3943. [PubMed] [Google Scholar]
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Chen S. S., Stanescu G., Magalski A. E., Qian Y. Y. Cyclosporin A is an adjuvant in murine IgE antibody responses. J Immunol. 1989 Jun 15;142(12):4225–4232. [PubMed] [Google Scholar]
- Coffman R. L., Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986 Feb 1;136(3):949–954. [PubMed] [Google Scholar]
- Diaz-Sanchez D., Kemeny D. M. The sensitivity of rat CD8+ and CD4+ T cells to ricin in vivo and in vitro and their relationship to IgE regulation. Immunology. 1990 Jan;69(1):71–77. [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Snapper C. M., Ohara J., Paul W. E. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9675–9678. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick O. L., Brooks D. L. Immunoglobulin E antibodies to pollens augmented in dogs by virus vaccines. Am J Vet Res. 1983 Mar;44(3):440–445. [PubMed] [Google Scholar]
- Hamaoka T., Katz D. H., Benacerraf B. Hapten-specific IgE antibody responses in mice. II. Cooperative interactions between adoptively transferred T and B lymphocytes in the development of IgE response. J Exp Med. 1973 Sep 1;138(3):538–556. doi: 10.1084/jem.138.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Batty J. E., Turner K. J. Inhibition of specific IgE responses in mice by pre-exposure to inhaled antigen. Immunology. 1981 Mar;42(3):409–417. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Sedgwick J. D., O'Leary C., Krska K., Leivers S. Long-lived IgE- and IgG-secreting cells in rodents manifesting persistent antibody responses. Cell Immunol. 1984 Dec;89(2):281–289. doi: 10.1016/0008-8749(84)90330-7. [DOI] [PubMed] [Google Scholar]
- Jarrett E. E., Hall E. Regulation of ongoing IgE antibody responses with minute doses antigen. Eur J Immunol. 1981 Jun;11(6):520–523. doi: 10.1002/eji.1830110615. [DOI] [PubMed] [Google Scholar]
- Katona I. M., Urban J. F., Jr, Finkelman F. D. The role of L3T4+ and Lyt-2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis. J Immunol. 1988 May 1;140(9):3206–3211. [PubMed] [Google Scholar]
- Katz D. H. Recent studies on the regulation of IgE antibody synthesis in experimental animals and man. Immunology. 1980 Sep;41(1):1–24. [PMC free article] [PubMed] [Google Scholar]
- Kemeny D. M., Frankland A. W., Fahkri Z. I., Trull A. K. Allergy to castor bean in the Sudan: measurement of serum IgE and specific IgE antibodies. Clin Allergy. 1981 Sep;11(5):463–471. doi: 10.1111/j.1365-2222.1981.tb01620.x. [DOI] [PubMed] [Google Scholar]
- Kemeny D. M., Harries M. G., Youlten L. J., Mackenzie-Mills M., Lessof M. H. Antibodies to purified bee venom proteins and peptides. I. Development of a highly specific RAST for bee venom antigens and its application to bee sting allergy. J Allergy Clin Immunol. 1983 May;71(5):505–514. doi: 10.1016/0091-6749(83)90469-4. [DOI] [PubMed] [Google Scholar]
- Kemeny D. M., Lessof M. H. Immune response to bee venom. II. Quantitation of the absolute amounts of IgE and IgG antibodies by saturation analysis. Int Arch Allergy Appl Immunol. 1987;83(2):113–120. [PubMed] [Google Scholar]
- Kemeny D. M., Richards D. Increased speed and sensitivity, and reduced sample size of a micro-radioallergosorbent test (MRAST). J Immunol Methods. 1988 Apr 6;108(1-2):105–113. doi: 10.1016/0022-1759(88)90408-5. [DOI] [PubMed] [Google Scholar]
- Kemeny D. M., Urbanek R., Samuel D., Richards D. Improved sensitivity and specificity of sandwich, competitive and capture enzyme-linked immunosorbent assays for allergen-specific antibodies. Int Arch Allergy Appl Immunol. 1985;77(1-2):198–200. doi: 10.1159/000233785. [DOI] [PubMed] [Google Scholar]
- Kemeny D. M., West F. B. Rapid washing of filter paper discs in a solid-phase radioimmunoassay with a constant flow washing device. J Immunol Methods. 1982;49(1):89–95. doi: 10.1016/0022-1759(82)90369-6. [DOI] [PubMed] [Google Scholar]
- Larsen G. L., Henson P. M. Mediators of inflammation. Annu Rev Immunol. 1983;1:335–359. doi: 10.1146/annurev.iy.01.040183.002003. [DOI] [PubMed] [Google Scholar]
- Larsen G. L., Henson P. M. Mediators of inflammation. Annu Rev Immunol. 1983;1:335–359. doi: 10.1146/annurev.iy.01.040183.002003. [DOI] [PubMed] [Google Scholar]
- ORDMAN D. An outbreak of bronchial asthma in South Africa, affecting more than 200 persons, caused by castor bean dust from an oil-processing factory. Int Arch Allergy Appl Immunol. 1955;7(1):10–24. doi: 10.1159/000228201. [DOI] [PubMed] [Google Scholar]
- Okumura K., Tada T. Regulation of homocytotropic antibody formation in the rat. VI. Inhibitory effect of thymocytes on the homocytotropic antibody response. J Immunol. 1971 Dec;107(6):1682–1689. [PubMed] [Google Scholar]
- PANZANI R. Respiratory castor bean dust allergy in the South of France with special reference to Marseilles. Int Arch Allergy Appl Immunol. 1957;11(3-4):224–236. doi: 10.1159/000228420. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. Induction of IgE-secreting cells and IgE isotype-specific suppressor T cells in the respiratory lymph nodes of rats in response to antigen inhalation. Cell Immunol. 1985 Aug;94(1):182–194. doi: 10.1016/0008-8749(85)90095-4. [DOI] [PubMed] [Google Scholar]
- Sopori M. L., Perrone R. S., Cherian S., Cross R. J., Kaplan A. M. Immunoregulation in the rat: characteristics of a suppressor T cell that inhibits antigen-dependent cell proliferation. J Immunol. 1985 Jul;135(1):80–86. [PubMed] [Google Scholar]
- Taniguchi M., Tada T. Regulation of homocytotropic antibody formation in the rat. IV. Effects of various immunosuppressive drugs. J Immunol. 1971 Aug;107(2):579–585. [PubMed] [Google Scholar]
- Thorpe S. C., Kemeny D. M., Panzani R., Lessof M. H. Allergy to castor bean. I. Its relationship to sensitization to common inhalant allergens (atopy). J Allergy Clin Immunol. 1988 Jul;82(1):62–66. doi: 10.1016/0091-6749(88)90052-8. [DOI] [PubMed] [Google Scholar]
- Thorpe S. C., Kemeny D. M., Panzani R., Lessof M. H. The relationship between total serum IgE and castor-bean-specific IgE antibodies in castor-bean-sensitive patients from Marseilles. Int Arch Allergy Appl Immunol. 1987;82(3-4):456–460. doi: 10.1159/000234253. [DOI] [PubMed] [Google Scholar]
- Thorpe S. C., Murdoch R. D., Kemeny D. M. The effect of the castor bean toxin, ricin, on rat IgE and IgG responses. Immunology. 1989 Nov;68(3):307–311. [PMC free article] [PubMed] [Google Scholar]
- Vaz E. M., Vaz N. M., Levine B. B. Persistent formation of reagins in mice injected with low doses of ovalbuminl. Immunology. 1971 Jul;21(1):11–15. [PMC free article] [PubMed] [Google Scholar]
- Wang B. S., Heacock E. H., Collins K. H., Hutchinson I. F., Tilney N. L., Mannick J. A. Suppressive effects of cyclosporin A on the induction of alloreactivity in vitro and in vivo. J Immunol. 1981 Jul;127(1):89–93. [PubMed] [Google Scholar]
- Watanabe N., Kojima S., Ovary Z. Suppression of IgE antibody production in SJL mice. I. Nonspecific suppressor T cells. J Exp Med. 1976 Apr 1;143(4):833–845. doi: 10.1084/jem.143.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]


