Abstract
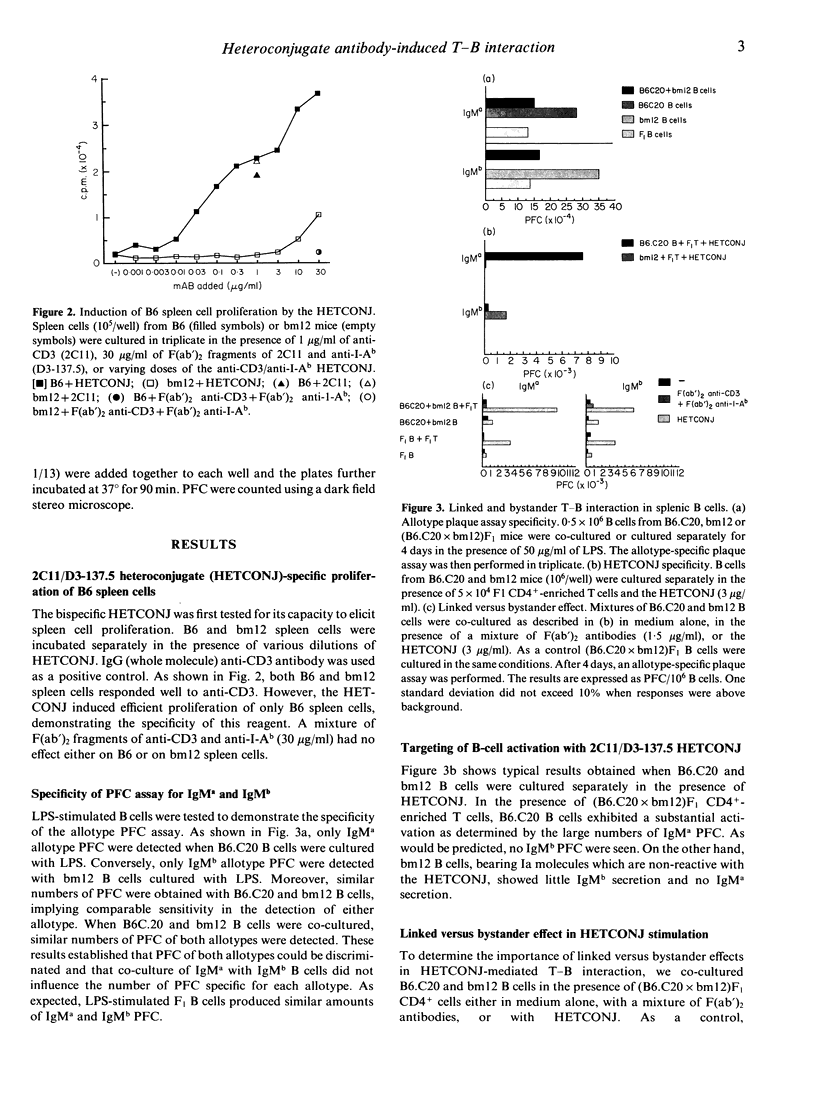
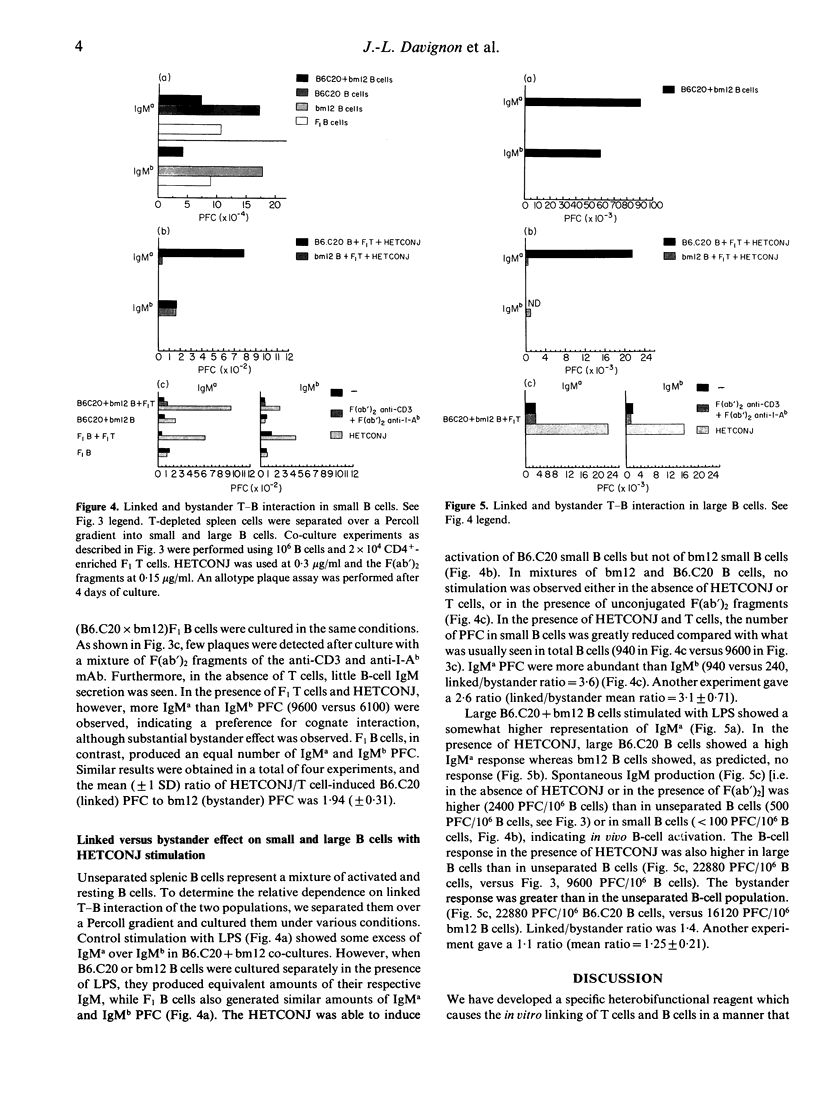
We have deliberately targeted collaboration between T cells and certain B cells by using a heteroconjugate (HETCONJ) antibody. This specific reagent was created by cross-linking the F(ab')2 portions of anti-I-Ab and anti-CD3 monoclonal antibodies. Spleen cells from B6 (I-Ab) but not bm12 (I-Abm12) mice proliferated in vitro in the presence of the HETCONJ. Similarly, T-cell dependent IgM secretion was induced in B cells from B6, yet only weakly in B cells from bm 12 mice. Using B cells from Igh allotype double congenic (B6.C20 Igha/I-Ab and bm12, Ighb/I-Abm12) mice in co-culture experiments, we have used the HETCONJ to study linked versus bystander T-B interaction. B-cell activation, mediated by HETCONJ, was 10 times greater in unseparated than in resting splenic B cells. T-B interaction through T-B contact was more efficient than activation through bystander effects both for unseparated and resting splenic B cells. Large, already activated B cells, in contrast, did not show a preference for linked recognition. Our reagent has mimicked some of the events involved in T-B collaboration and may be useful in studying the molecular basis of cellular interactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Matsueda G. R., Evans R. J., Dunbar J. B., Jr, Marshall G. R., Unanue E. R. Identification of the T-cell and Ia contact residues of a T-cell antigenic epitope. 1987 Jun 25-Jul 1Nature. 327(6124):713–715. doi: 10.1038/327713a0. [DOI] [PubMed] [Google Scholar]
- Cambier J. C., Lehmann K. R. Ia-mediated signal transduction leads to proliferation of primed B lymphocytes. J Exp Med. 1989 Sep 1;170(3):877–886. doi: 10.1084/jem.170.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Cohen P. L., Rapoport R. G., Eisenberg R. A. Hidden autoantibodies against common serum proteins in murine systemic lupus erythematosus. Detection by in vitro plaque-forming cell assay. J Exp Med. 1985 Jun 1;161(6):1587–1592. doi: 10.1084/jem.161.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davignon J. L., Cohen P. L., Eisenberg R. A. Rapid T cell receptor modulation accompanies lack of in vitro mitogenic responsiveness of double negative T cells to anti-CD3 monoclonal antibody in MRL/Mp-lpr mice. J Immunol. 1988 Sep 15;141(6):1848–1854. [PubMed] [Google Scholar]
- Defrance T., Aubry J. P., Vanbervliet B., Banchereau J. Human interferon-gamma acts as a B cell growth factor in the anti-IgM antibody co-stimulatory assay but has no direct B cell differentiation activity. J Immunol. 1986 Dec 15;137(12):3861–3867. [PubMed] [Google Scholar]
- Defranco A. L., Raveche E. S., Asofsky R., Paul W. E. Frequency of B lymphocytes responsive to anti-immunoglobulin. J Exp Med. 1982 May 1;155(5):1523–1536. doi: 10.1084/jem.155.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Paul W. E. Regulation of B-cell growth and differentiation by soluble factors. Annu Rev Immunol. 1983;1:307–333. doi: 10.1146/annurev.iy.01.040183.001515. [DOI] [PubMed] [Google Scholar]
- Jensen P. E., Kapp J. A. Bystander help in primary immune responses in vivo. J Exp Med. 1986 Sep 1;164(3):841–854. doi: 10.1084/jem.164.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. Cooperation between T and B cells. A minimal model. Immunol Rev. 1987 Oct;99:5–18. doi: 10.1111/j.1600-065x.1987.tb01169.x. [DOI] [PubMed] [Google Scholar]
- Jung G., Honsik C. J., Reisfeld R. A., Müller-Eberhard H. J. Activation of human peripheral blood mononuclear cells by anti-T3: killing of tumor target cells coated with anti-target-anti-T3 conjugates. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4479–4483. doi: 10.1073/pnas.83.12.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H., Rolink A., Melchers F. Recombinant interleukin 2 or 5, but not 3 or 4, induces maturation of resting mouse B lymphocytes and propagates proliferation of activated B cell blasts. J Exp Med. 1988 Apr 1;167(4):1377–1390. doi: 10.1084/jem.167.4.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpovsky B., Titus J. A., Stephany D. A., Segal D. M. Production of target-specific effector cells using hetero-cross-linked aggregates containing anti-target cell and anti-Fc gamma receptor antibodies. J Exp Med. 1984 Dec 1;160(6):1686–1701. doi: 10.1084/jem.160.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura H., Berzofsky J. A. Enhancement of antigenic potency in vitro and immunogenicity in vivo by coupling the antigen to anti-immunoglobulin. J Immunol. 1986 Jan;136(1):58–65. [PubMed] [Google Scholar]
- Kung J. T., Sharrow S. O., Sieckmann D. G., Lieberman R., Paul W. E. A mouse IgM allotypic determinant (Igh-6.5) recognized by a monoclonal rat antibody. J Immunol. 1981 Sep;127(3):873–876. [PubMed] [Google Scholar]
- Kupfer A., Swain S. L., Singer S. J. The specific direct interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J Exp Med. 1987 Jun 1;165(6):1565–1580. doi: 10.1084/jem.165.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison N. A. The carrier effect in the secondary response to hapten-protein conjugates. II. Cellular cooperation. Eur J Immunol. 1971 Jan;1(1):18–27. doi: 10.1002/eji.1830010104. [DOI] [PubMed] [Google Scholar]
- Moingeon P., Chang H. C., Wallner B. P., Stebbins C., Frey A. Z., Reinherz E. L. CD2-mediated adhesion facilitates T lymphocyte antigen recognition function. Nature. 1989 May 25;339(6222):312–314. doi: 10.1038/339312a0. [DOI] [PubMed] [Google Scholar]
- Mosier D. E. T cell activation of antigen-specific antibody responses by large B cells is MHC restricted. J Immunol. 1986 Mar 15;136(6):2090–2094. [PubMed] [Google Scholar]
- Newell M. K., Haughn L. J., Maroun C. R., Julius M. H. Death of mature T cells by separate ligation of CD4 and the T-cell receptor for antigen. Nature. 1990 Sep 20;347(6290):286–289. doi: 10.1038/347286a0. [DOI] [PubMed] [Google Scholar]
- Noelle R. J., Snow E. C. Cognate interactions between helper T cells and B cells. Immunol Today. 1990 Oct;11(10):361–368. doi: 10.1016/0167-5699(90)90142-v. [DOI] [PubMed] [Google Scholar]
- Noelle R. J., Snow E. C., Uhr J. W., Vitetta E. S. Activation of antigen-specific B cells: role of T cells, cytokines, and antigen in induction of growth and differentiation. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6628–6631. doi: 10.1073/pnas.80.21.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettgen H. C., Terhorst C. A review of the structure and function of the T-cell receptor-T3 complex. Crit Rev Immunol. 1987;7(2):131–167. [PubMed] [Google Scholar]
- Oliver K., Noelle R. J., Uhr J. W., Krammer P. H., Vitetta E. S. B-cell growth factor (B-cell growth factor I or B-cell-stimulating factor, provisional 1) is a differentiation factor for resting B cells and may not induce cell growth. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2465–2467. doi: 10.1073/pnas.82.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T. A noncognate interaction with anti-receptor antibody-activated helper T cells induces small resting murine B cells to proliferate and to secrete antibody. Eur J Immunol. 1988 Mar;18(3):395–401. doi: 10.1002/eji.1830180312. [DOI] [PubMed] [Google Scholar]
- Ozaki S., Berzofsky J. A. Antibody conjugates mimic specific B cell presentation of antigen: relationship between T and B cell specificity. J Immunol. 1987 Jun 15;138(12):4133–4142. [PubMed] [Google Scholar]
- Perez P., Hoffman R. W., Titus J. A., Segal D. M. Specific targeting of human peripheral blood T cells by heteroaggregates containing anti-T3 crosslinked to anti-target cell antibodies. J Exp Med. 1986 Jan 1;163(1):166–178. doi: 10.1084/jem.163.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo W. J., Conrad L., Janeway C. A., Jr Receptor-directed focusing of lymphokine release by helper T cells. Nature. 1988 Mar 24;332(6162):378–380. doi: 10.1038/332378a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen R., Takatsu K., Harada N., Takahashi T., Bottomly K. T cell-dependent hapten-specific and polyclonal B cell responses require release of interleukin 5. J Immunol. 1988 Feb 1;140(3):705–712. [PubMed] [Google Scholar]
- Rubin B., Boned A. Insulin-specific T cell help to TNP-specific B cells requires activation via the antigen T cell receptor. Eur J Immunol. 1986 Oct;16(10):1223–1229. doi: 10.1002/eji.1830161007. [DOI] [PubMed] [Google Scholar]
- Sanders V. M., Snyder J. M., Uhr J. W., Vitetta E. S. Characterization of the physical interaction between antigen-specific B and T cells. J Immunol. 1986 Oct 15;137(8):2395–2404. [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Sarmiento M., Loken M. R., Fitch F. W. Structural differences in cell surface T25 polypeptides from thymocytes and cloned T cells. Hybridoma. 1981;1(1):13–26. doi: 10.1089/hyb.1.1981.1.13. [DOI] [PubMed] [Google Scholar]
- Scherle P. A., Gerhard W. Functional analysis of influenza-specific helper T cell clones in vivo. T cells specific for internal viral proteins provide cognate help for B cell responses to hemagglutinin. J Exp Med. 1986 Oct 1;164(4):1114–1128. doi: 10.1084/jem.164.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Snider D. P., Segal D. M. Targeted antigen presentation using crosslinked antibody heteroaggregates. J Immunol. 1987 Sep 1;139(5):1609–1616. [PubMed] [Google Scholar]
- Sprent J. Role of H-2 gene products in the function of T helper cells from normal and chimeric mice in vivo. Immunol Rev. 1978;42:108–137. doi: 10.1111/j.1600-065x.1978.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Yewdell J. W., Bevan M. J. Hybrid antibody-mediated lysis of virus-infected cells. Eur J Immunol. 1987 Apr;17(4):571–574. doi: 10.1002/eji.1830170422. [DOI] [PubMed] [Google Scholar]
- Stall A. M., Loken M. R. Allotypic specificities of murine IgD and IgM recognized by monoclonal antibodies. J Immunol. 1984 Feb;132(2):787–795. [PubMed] [Google Scholar]
- Zepp F., Staerz U. D. Thymic selection process induced by hybrid antibodies. Nature. 1988 Dec 1;336(6198):473–475. doi: 10.1038/336473a0. [DOI] [PubMed] [Google Scholar]


