Abstract
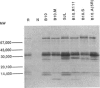
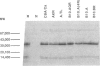
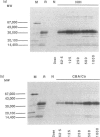
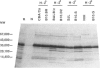
Humans infected with the parasitic nematode Trichinella spiralis vary in the specificity of their antibody responses to the antigens of the parasite. The possibility that such host variation in antigen recognition has a genetic basis was examined in infected inbred mice whose antigen recognition profiles were characterized by immunoprecipitation of biosynthetically labelled secreted materials of adult parasites and SDS-PAGE. The strains varied considerably in repertoire and none produced detectable antibody to all the potential antigens. Using a panel of H-2 congenic and recombinant strains it was established that the repertoire was determined by the major histocompatibility complex (MHC), the I-A region in particular. Other factors, such as level of infection and variation between individuals, affected antigen recognition profiles, but this was always within limits imposed by the MHC. Lastly, an attempt to correlate antibody repertoire with relative susceptibility or resistance to T. spiralis failed to reveal any clear association. This also applied to the AKR/J and AKR-Fv-1b strains, which are H-2-identical but differ in a non-MHC susceptibility locus. These findings would argue, therefore, that the I-A region controls the antibody repertoire in this nematode infection but that the repertoire overall has little influence on the efficiency with which the infections are controlled by the immune system. Should this also apply for other nematode infections, then antigen recognition profiles of infected individual humans and domestic animals might not, therefore, be useful indicators of relative resistance or susceptibility to infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almond N. M., Parkhouse R. M., Chapa-Ruiz M. R., Garcia-Ortigoza E. The response of humans to surface and secreted antigens of Trichinella spiralis. Trop Med Parasitol. 1986 Dec;37(4):381–384. [PubMed] [Google Scholar]
- Barnett B. C., Graham C. M., Burt D. S., Skehel J. J., Thomas D. B. The immune response of BALB/c mice to influenza hemagglutinin: commonality of the B cell and T cell repertoires and their relevance to antigenic drift. Eur J Immunol. 1989 Mar;19(3):515–521. doi: 10.1002/eji.1830190316. [DOI] [PubMed] [Google Scholar]
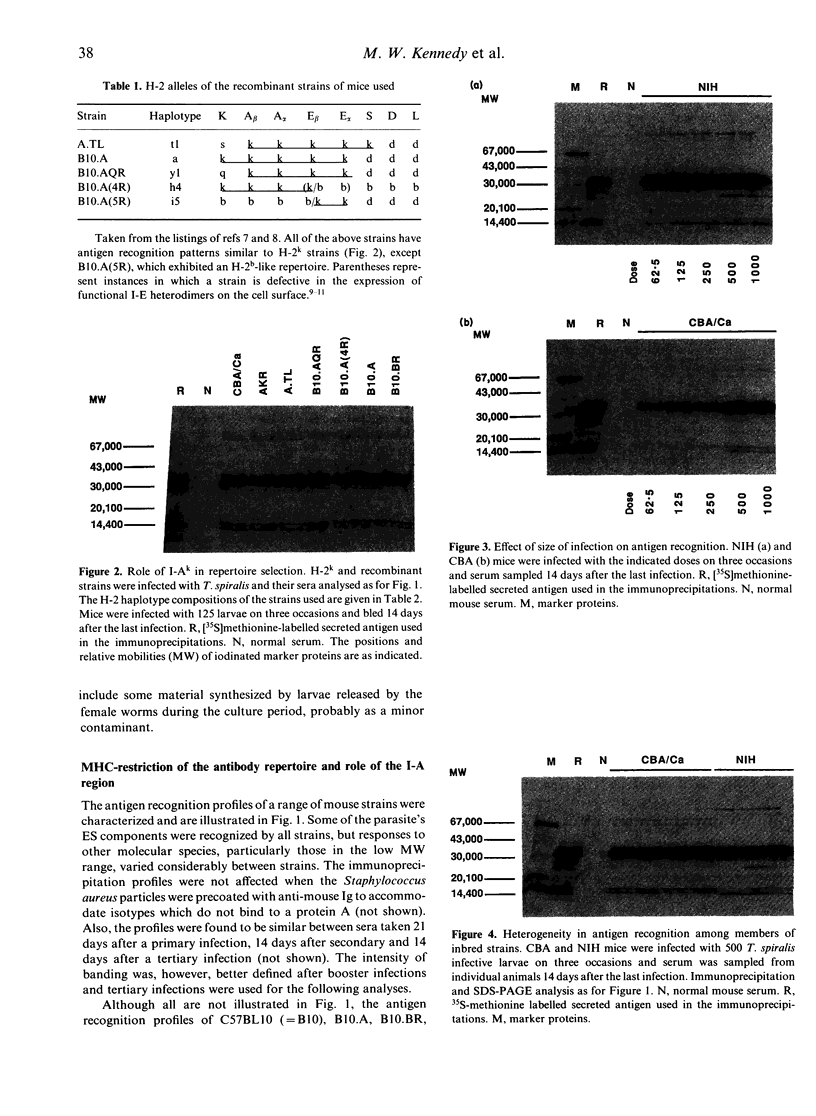
- Else K. J., Wakelin D., Wassom D. L., Hauda K. M. MHC-restricted antibody responses to Trichuris muris excretory/secretory (E/S) antigen. Parasite Immunol. 1990 Sep;12(5):509–527. doi: 10.1111/j.1365-3024.1990.tb00985.x. [DOI] [PubMed] [Google Scholar]
- Else K., Wakelin D. Genetic variation in the humoral immune responses of mice to the nematode Trichuris muris. Parasite Immunol. 1989 Jan;11(1):77–90. doi: 10.1111/j.1365-3024.1989.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Germain R. N. Immunology. Making a molecular match. Nature. 1990 Mar 1;344(6261):19–22. doi: 10.1038/344019a0. [DOI] [PubMed] [Google Scholar]
- Graham C. M., Barnett B. C., Hartlmayr I., Burt D. S., Faulkes R., Skehel J. J., Thomas D. B. The structural requirements for class II (I-Ad)-restricted T cell recognition of influenza hemagglutinin: B cell epitopes define T cell epitopes. Eur J Immunol. 1989 Mar;19(3):523–528. doi: 10.1002/eji.1830190317. [DOI] [PubMed] [Google Scholar]
- Grencis R. K., Crawford C., Pritchard D. I., Behnke J. M., Wakelin D. Immunization of mice with surface antigens from the muscle larvae of Trichinella spiralis. Parasite Immunol. 1986 Nov;8(6):587–596. doi: 10.1111/j.1365-3024.1986.tb00872.x. [DOI] [PubMed] [Google Scholar]
- Grencis R. K., Riedlinger J., Wakelin D. L3T4-positive T lymphoblasts are responsible for transfer of immunity to Trichinella spiralis in mice. Immunology. 1985 Oct;56(2):213–218. [PMC free article] [PubMed] [Google Scholar]
- Harris D. P., Bäckström B. T., Booth R. J., Love S. G., Harding D. R., Watson J. D. The mapping of epitopes of the 18-kDa protein of Mycobacterium leprae recognized by murine T cells in a proliferation assay. J Immunol. 1989 Sep 15;143(6):2006–2012. [PubMed] [Google Scholar]
- Hayes C. E., Klyczek K. K., Krum D. P., Whitcomb R. M., Hullett D. A., Cantor H. Chromosome 4 Jt gene controls murine T cell surface I-J expression. Science. 1984 Feb 10;223(4636):559–563. doi: 10.1126/science.6607530. [DOI] [PubMed] [Google Scholar]
- Jacqueline E., Vernes A., Bout D., Biguet J. Trichinella spiralis: facteurs immunitaires inhibiteurs de la production de larves. II. Première analyse in vitro des facteurs humoraux et sécrétoires actifs chez la souris, le rat, et le miniporc infestés ou immunisés. Exp Parasitol. 1978 Jun;45(1):42–54. doi: 10.1016/0014-4894(78)90043-7. [DOI] [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., McDevitt H. O. Two-gene control of the expression of a murine Ia antigen. J Exp Med. 1978 Oct 1;148(4):925–939. doi: 10.1084/jem.148.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., McDevitt H. O. Variable synthesis and expression of E alpha and Ae (E beta) Ia polypeptide chains in mice of different H-2 haplotypes. Immunogenetics. 1981;12(3-4):321–337. doi: 10.1007/BF01561674. [DOI] [PubMed] [Google Scholar]
- Jungery M., Ogilvie B. M. Antibody response to stage-specific Trichinella spiralis surface antigens in strong and weak responder mouse strains. J Immunol. 1982 Aug;129(2):839–843. [PubMed] [Google Scholar]
- Kee K. C., Taylor D. W., Cordingley J. S., Butterworth A. E., Munro A. J. Genetic influence on the antibody response to antigens of Schistosoma mansoni in chronically infected mice. Parasite Immunol. 1986 Nov;8(6):565–574. doi: 10.1111/j.1365-3024.1986.tb00870.x. [DOI] [PubMed] [Google Scholar]
- Kennedy M. W. Effects of the host immune response on the longevity, fecundity and position in the intestine of Trichinella spiralis in mice. Parasitology. 1980 Feb;80(1):49–60. doi: 10.1017/s0031182000000500. [DOI] [PubMed] [Google Scholar]
- Kennedy M. W. Genetic control of the immune repertoire in nematode infections. Parasitol Today. 1989 Oct;5(10):316–324. doi: 10.1016/0169-4758(89)90122-1. [DOI] [PubMed] [Google Scholar]
- Kennedy M. W., Gordon A. M., Tomlinson L. A., Qureshi F. Genetic (major histocompatibility complex?) control of the antibody repertoire to the secreted antigens of Ascaris. Parasite Immunol. 1987 Mar;9(2):269–273. doi: 10.1111/j.1365-3024.1987.tb00506.x. [DOI] [PubMed] [Google Scholar]
- Kennedy M. W., McIntosh A. E., Blair A. J., McLaughlin D. MHC (RT1) restriction of the antibody repertoire to infection with the nematode Nippostrongylus brasiliensis in the rat. Immunology. 1990 Nov;71(3):317–322. [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. W., Qureshi F. Stage-specific secreted antigens of the parasitic larval stages of the nematode Ascaris. Immunology. 1986 Jul;58(3):515–522. [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. W., Tomlinson L. A., Fraser E. M., Christie J. F. The specificity of the antibody response to internal antigens of Ascaris: heterogeneity in infected humans, and MHC (H-2) control of the repertoire in mice. Clin Exp Immunol. 1990 May;80(2):219–224. doi: 10.1111/j.1365-2249.1990.tb05237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J., Figueroa F., David C. S. H-2 haplotypes, genes and antigens: second listing. II. The H-2 complex. Immunogenetics. 1983;17(6):553–596. doi: 10.1007/BF00366126. [DOI] [PubMed] [Google Scholar]
- Kwan-Lim G. E., Maizels R. M. MHC and non-MHC-restricted recognition of filarial surface antigens in mice transplanted with adult Brugia malayi parasites. J Immunol. 1990 Sep 15;145(6):1912–1920. [PubMed] [Google Scholar]
- Lockyer M. J., Marsh K., Newbold C. I. Wild isolates of Plasmodium falciparum show extensive polymorphism in T cell epitopes of the circumsporozoite protein. Mol Biochem Parasitol. 1989 Dec;37(2):275–280. doi: 10.1016/0166-6851(89)90159-x. [DOI] [PubMed] [Google Scholar]
- Matzinger P. A one-receptor view of T-cell behaviour. Nature. 1981 Aug 6;292(5823):497–501. doi: 10.1038/292497a0. [DOI] [PubMed] [Google Scholar]
- McLaren A., Tait A. Cytoplasmic isocitrate dehydrogenase variation within the C3H inbred strain. Genet Res. 1969 Aug;14(1):93–94. doi: 10.1017/s0016672300001890. [DOI] [PubMed] [Google Scholar]
- Miller H. R. The protective mucosal response against gastrointestinal nematodes in ruminants and laboratory animals. Vet Immunol Immunopathol. 1984 May;6(1-2):167–259. doi: 10.1016/0165-2427(84)90051-5. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Jones P. P., Loken M. R., McDevitt H. O. Interaction between I region loci influences the expression of a cell surface Ia antigen. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5404–5408. doi: 10.1073/pnas.77.9.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond L., Wassom D. L., Hayes C. E. Evidence for differential induction of helper T cell subsets during Trichinella spiralis infection. J Immunol. 1989 Dec 15;143(12):4232–4237. [PubMed] [Google Scholar]
- Riedlinger J., Grencis R. K., Wakelin D. Antigen-specific T-cell lines transfer protective immunity against Trichinella spiralis in vivo. Immunology. 1986 May;58(1):57–61. [PMC free article] [PubMed] [Google Scholar]
- Rittner C., Schneider P. M. Complexity of MHC class III genes and complement polymorphism. Immunol Today. 1989 Dec;10(12):401–403. doi: 10.1016/0167-5699(89)90034-0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A clonal deletion model for Ir gene control of the immune response. Scand J Immunol. 1978;7(1):3–10. doi: 10.1111/j.1365-3083.1978.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Thomas D. B., Skehel J. J., Mills K. H., Graham C. M. Suicide selection of murine T helper clones specific for variable regions of the influenza hemagglutinin molecule. Eur J Immunol. 1986 Jul;16(7):789–793. doi: 10.1002/eji.1830160712. [DOI] [PubMed] [Google Scholar]
- Tomlinson L. A., Christie J. F., Fraser E. M., McLaughlin D., McIntosh A. E., Kennedy M. W. MHC restriction of the antibody repertoire to secretory antigens, and a major allergen, of the nematode parasite Ascaris. J Immunol. 1989 Oct 1;143(7):2349–2356. [PubMed] [Google Scholar]
- Vidović D., Matzinger P. Unresponsiveness to a foreign antigen can be caused by self-tolerance. Nature. 1988 Nov 17;336(6196):222–225. doi: 10.1038/336222a0. [DOI] [PubMed] [Google Scholar]
- Wassom D. L., Brooks B. O., Cypess R. H., David C. S. A survey of susceptibility to infection with Trichinella spiralis of inbred mouse strains sharing common H-2 alleles but different genetic backgrounds. J Parasitol. 1983 Dec;69(6):1033–1037. [PubMed] [Google Scholar]
- Wassom D. L., Wakelin D., Brooks B. O., Krco C. J., David C. S. Genetic control of immunity to Trichinella spiralis infections of mice. Hypothesis to explain the role of H-2 genes in primary and challenge infections. Immunology. 1984 Apr;51(4):625–631. [PMC free article] [PubMed] [Google Scholar]