Abstract
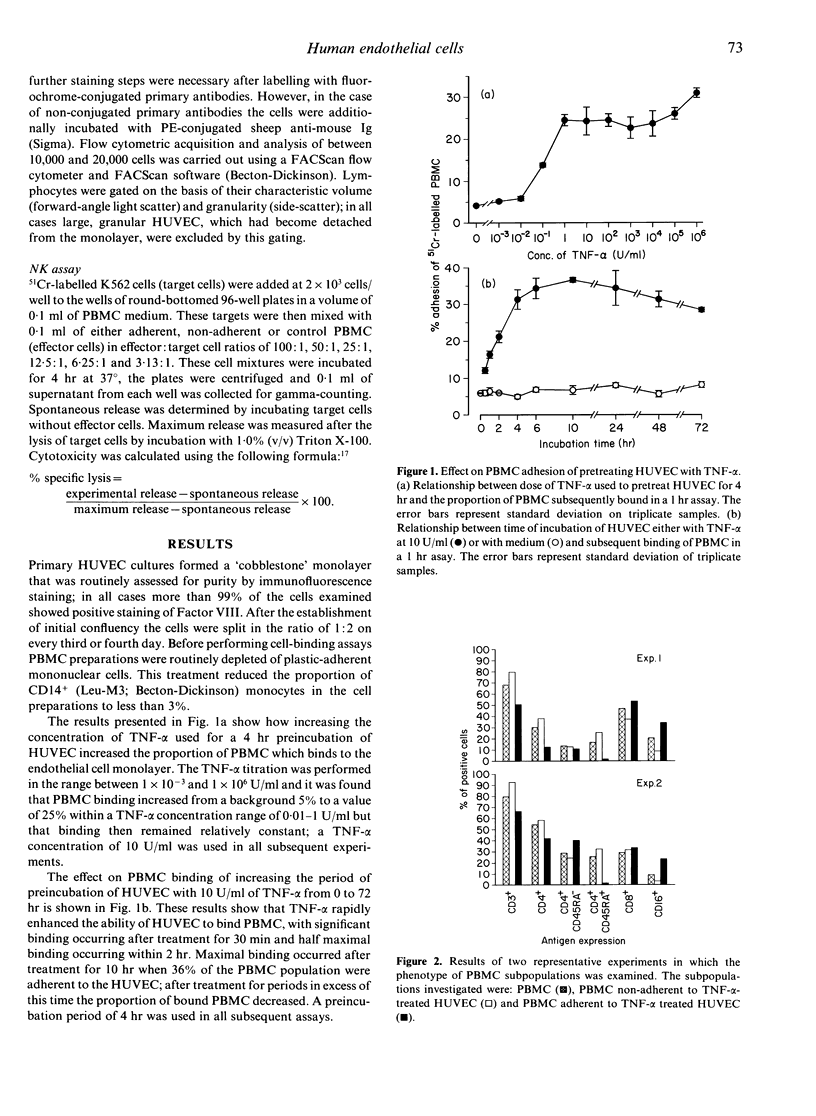
Human umbilical vein endothelial cells (HUVEC) were cultured and treated for varying periods with a range of concentrations of tumour necrosis factor-alpha (TNF-alpha). After this treatment the proportion of peripheral blood mononuclear cells (PBMC), previously depleted of plastic adherent cells, capable of binding to the endothelial cells was assessed. Few PBMC bound to HUVEC which had not been pretreated with TNF-alpha but up to 36% bound after pretreatment of the endothelial cells with TNF-alpha for 10 hr at a concentration of 10 U/ml. Phenotypic characterization of the adherent and non-adherent PBMC subpopulations revealed that natural killer (NK) cells (CD16+) and a proportion of memory helper T cells (CD4+ CD45RA-) bound to TNF-alpha pretreated HUVEC but that few naive helper T cells (CD4+ CD45RA+) showed similar binding. Cytotoxicity assays for NK activity were used to analyse functionally the adherent and non-adherent PBMC subpopulations. It was found that the cell subpopulation which did not adhere to TNF-alpha pretreated HUVEC mediated little lysis of K562 target cells. Conversely, the endothelial cell-adherent PBMC subpopulation produced active lysis supporting the phenotypic evidence that NK cells were concentrated within this subpopulation. These results suggest that TNF-alpha has a rapid and profound up-regulatory effect on the expression of adhesion molecules on the surface of HUVEC. Furthermore, it is apparent that these up-regulated adhesion molecules preferentially bind NK cells and a subset of memory helper T cells from the PBMC population.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- Cavender D. E., Haskard D. O., Joseph B., Ziff M. Interleukin 1 increases the binding of human B and T lymphocytes to endothelial cell monolayers. J Immunol. 1986 Jan;136(1):203–207. [PubMed] [Google Scholar]
- Cavender D., Saegusa Y., Ziff M. Stimulation of endothelial cell binding of lymphocytes by tumor necrosis factor. J Immunol. 1987 Sep 15;139(6):1855–1860. [PubMed] [Google Scholar]
- Damle N. K., Doyle L. V. Ability of human T lymphocytes to adhere to vascular endothelial cells and to augment endothelial permeability to macromolecules is linked to their state of post-thymic maturation. J Immunol. 1990 Feb 15;144(4):1233–1240. [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kist A., Ho A. D., Räth U., Wiedenmann B., Bauer A., Schlick E., Kirchner H., Männel D. N. Decrease of natural killer cell activity and monokine production in peripheral blood of patients treated with recombinant tumor necrosis factor. Blood. 1988 Jul;72(1):344–348. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Munro J. M., Pober J. S., Cotran R. S. Tumor necrosis factor and interferon-gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am J Pathol. 1989 Jul;135(1):121–133. [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Ostensen M. E., Thiele D. L., Lipsky P. E. Tumor necrosis factor-alpha enhances cytolytic activity of human natural killer cells. J Immunol. 1987 Jun 15;138(12):4185–4191. [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Haskard D., Panayi G. The preferential accumulation of helper-inducer T lymphocytes in inflammatory lesions: evidence for regulation by selective endothelial and homotypic adhesion. Eur J Immunol. 1988 Sep;18(9):1397–1404. doi: 10.1002/eji.1830180915. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Schwartz B. R., Wayner E. A., Carlos T. M., Ochs H. D., Harlan J. M. Identification of surface proteins mediating adherence of CD11/CD18-deficient lymphoblastoid cells to cultured human endothelium. J Clin Invest. 1990 Jun;85(6):2019–2022. doi: 10.1172/JCI114668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Thornhill M. H., Haskard D. O. IL-4 regulates endothelial cell activation by IL-1, tumor necrosis factor, or IFN-gamma. J Immunol. 1990 Aug 1;145(3):865–872. [PubMed] [Google Scholar]
- Thornhill M. H., Kyan-Aung U., Haskard D. O. IL-4 increases human endothelial cell adhesiveness for T cells but not for neutrophils. J Immunol. 1990 Apr 15;144(8):3060–3065. [PubMed] [Google Scholar]
- Thornhill M. H., Kyan-Aung U., Lee T. H., Haskard D. O. T cells and neutrophils exhibit differential adhesion to cytokine-stimulated endothelial cells. Immunology. 1990 Feb;69(2):287–292. [PMC free article] [PubMed] [Google Scholar]
- Voth R., Rossol S., Gallati H., Pracht I., Laubenstein H. P., Hess G., Müller W. E., Schröder H. C., Jochum C., Meyer zum Büschenfelde K. H. In vivo and in vitro induction of natural killer cells by cloned human tumor necrosis factor. Cancer Immunol Immunother. 1988;27(2):128–132. doi: 10.1007/BF00200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellicome S. M., Thornhill M. H., Pitzalis C., Thomas D. S., Lanchbury J. S., Panayi G. S., Haskard D. O. A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumor necrosis factor, IL-1, or lipopolysaccharide. J Immunol. 1990 Apr 1;144(7):2558–2565. [PubMed] [Google Scholar]
- Yu C. L., Haskard D. O., Cavender D., Johnson A. R., Ziff M. Human gamma interferon increases the binding of T lymphocytes to endothelial cells. Clin Exp Immunol. 1985 Dec;62(3):554–560. [PMC free article] [PubMed] [Google Scholar]


