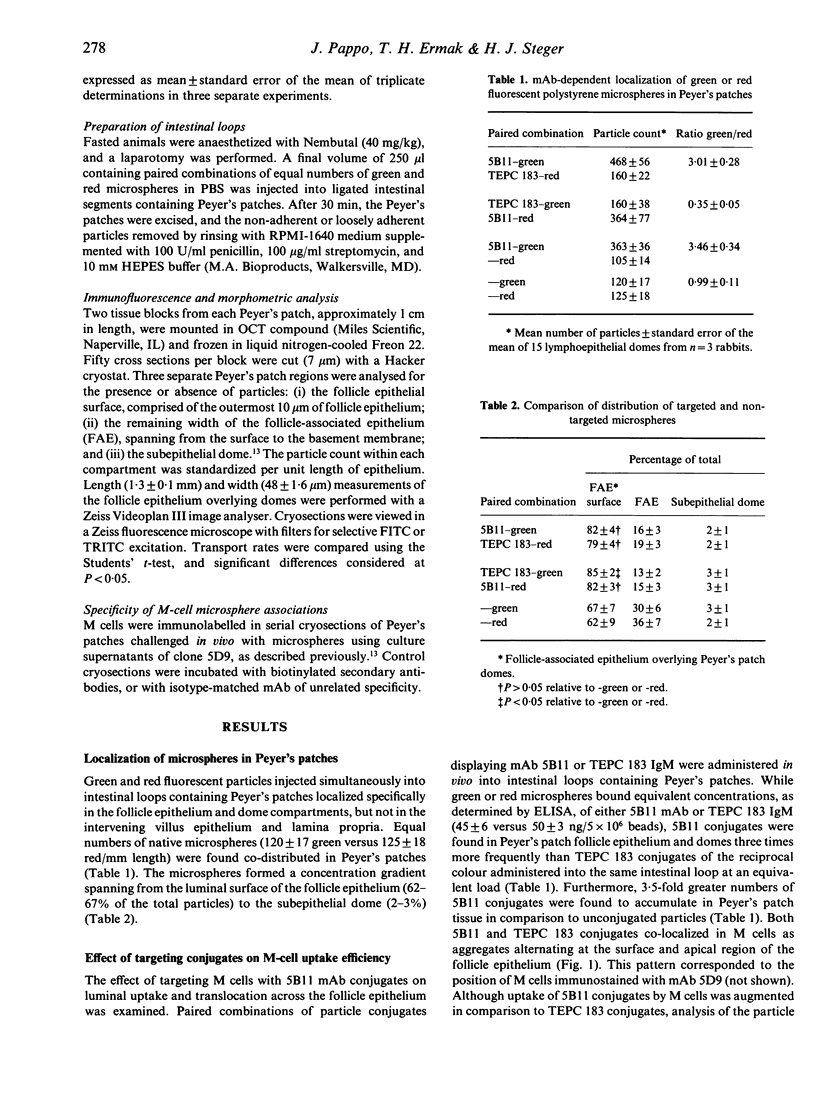
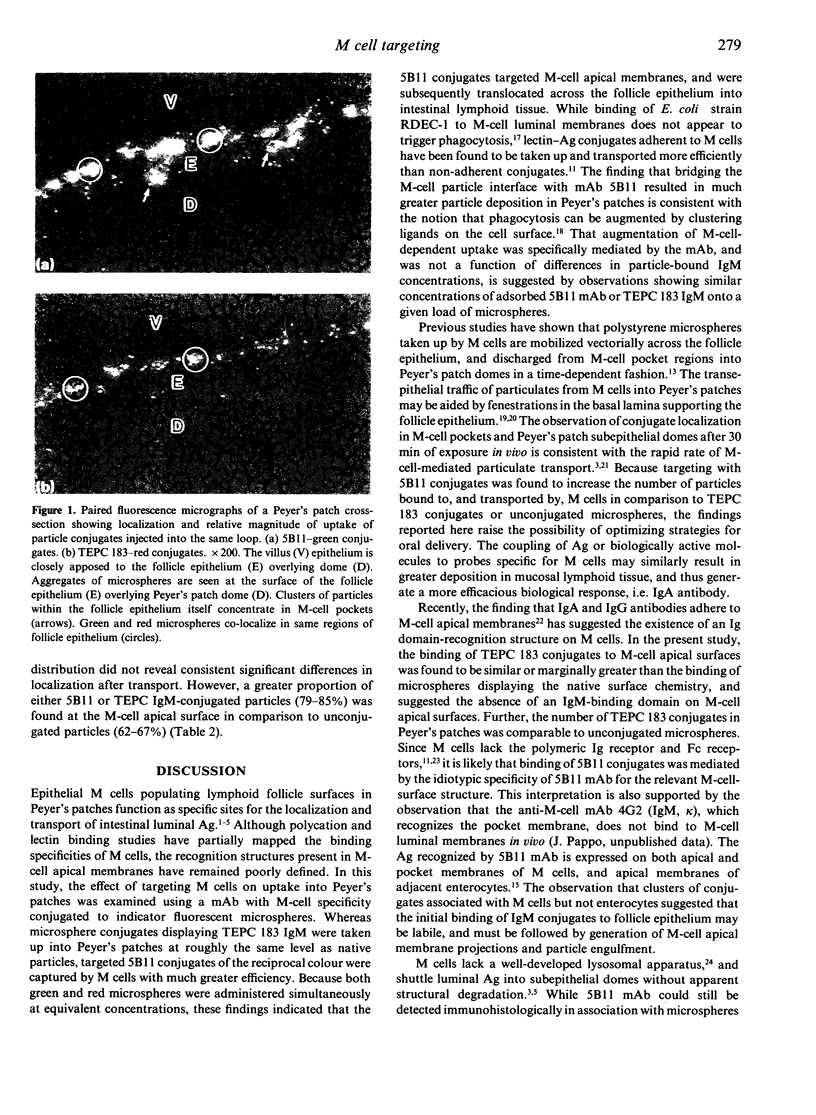
Abstract
The ability to deliver particulates to Peyer's patch M cells for uptake into gut-associated lymphoid tissue was examined by administering simultaneously fluorescent green and red polystyrene microspheres into NZW rabbit intestinal loops containing Peyer's patches. Whereas green and red microspheres were taken up by M cells at equivalent concentrations (120 +/- 17 versus 125 +/- 18/mm length of dome), particles conjugated to the anti-M-cell monoclonal antibody 5B11 (IgM, kappa) were internalized by M cells 3-3.5 times more efficiently than conjugates displaying IgM of unrelated specificity (TEPC 183) or native particles of the reciprocal colour inoculated into the same loop at a comparable load. The microspheres formed a concentration gradient from lumen to subepithelial dome, and localized on M-cell apical membranes, M-cell pockets, and subepithelial domes. The transport rate across M cells of 5B11 or TEPC 183 conjugates was similar to that of untreated microspheres. These observations show that intestinal uptake into Peyer's patches can be upregulated by targeting M-cell luminal membrane structures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjerke K., Brandtzaeg P., Fausa O. T cell distribution is different in follicle-associated epithelium of human Peyer's patches and villous epithelium. Clin Exp Immunol. 1988 Nov;74(2):270–275. [PMC free article] [PubMed] [Google Scholar]
- Bockman D. E., Cooper M. D. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer's patches. An electron microscopic study. Am J Anat. 1973 Apr;136(4):455–477. doi: 10.1002/aja.1001360406. [DOI] [PubMed] [Google Scholar]
- Bye W. A., Allan C. H., Trier J. S. Structure, distribution, and origin of M cells in Peyer's patches of mouse ileum. Gastroenterology. 1984 May;86(5 Pt 1):789–801. [PubMed] [Google Scholar]
- Ermak T. H., Steger H. J., Pappo J. Phenotypically distinct subpopulations of T cells in domes and M-cell pockets of rabbit gut-associated lymphoid tissues. Immunology. 1990 Dec;71(4):530–537. [PMC free article] [PubMed] [Google Scholar]
- Inman L. R., Cantey J. R. Specific adherence of Escherichia coli (strain RDEC-1) to membranous (M) cells of the Peyer's patch in Escherichia coli diarrhea in the rabbit. J Clin Invest. 1983 Jan;71(1):1–8. doi: 10.1172/JCI110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landsverk T. Phagocytosis and transcytosis by the follicle-associated epithelium of the ileal Peyer's patch in calves. Immunol Cell Biol. 1988 Aug;66(Pt 4):261–268. doi: 10.1038/icb.1988.35. [DOI] [PubMed] [Google Scholar]
- LeFevre M. E., Hammer R., Joel D. D. Macrophages of the mammalian small intestine: a review. J Reticuloendothel Soc. 1979 Nov;26(5):553–573. [PubMed] [Google Scholar]
- London S. D., Rubin D. H., Cebra J. J. Gut mucosal immunization with reovirus serotype 1/L stimulates virus-specific cytotoxic T cell precursors as well as IgA memory cells in Peyer's patches. J Exp Med. 1987 Mar 1;165(3):830–847. doi: 10.1084/jem.165.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcial M. A., Madara J. L. Cryptosporidium: cellular localization, structural analysis of absorptive cell-parasite membrane-membrane interactions in guinea pigs, and suggestion of protozoan transport by M cells. Gastroenterology. 1986 Mar;90(3):583–594. doi: 10.1016/0016-5085(86)91112-1. [DOI] [PubMed] [Google Scholar]
- McClugage S. G., Low F. N., Zimny M. L. Porosity of the basement membrane overlying Peyer's patches in rats and monkeys. Gastroenterology. 1986 Nov;91(5):1128–1133. doi: 10.1016/s0016-5085(86)80007-5. [DOI] [PubMed] [Google Scholar]
- Neutra M. R., Phillips T. L., Mayer E. L., Fishkind D. J. Transport of membrane-bound macromolecules by M cells in follicle-associated epithelium of rabbit Peyer's patch. Cell Tissue Res. 1987 Mar;247(3):537–546. doi: 10.1007/BF00215747. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Apple R. T., Bhalla D. K. Morphometric and cytochemical analysis of lysosomes in rat Peyer's patch follicle epithelium: their reduction in volume fraction and acid phosphatase content in M cells compared to adjacent enterocytes. Anat Rec. 1986 Dec;216(4):521–527. doi: 10.1002/ar.1092160409. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Bhalla D. K. Cytochemical analysis of alkaline phosphatase and esterase activities and of lectin-binding and anionic sites in rat and mouse Peyer's patch M cells. Am J Anat. 1983 Oct;168(2):199–212. doi: 10.1002/aja.1001680207. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Pierce N. F., Apple R. T., Cray W. C., Jr M cell transport of Vibrio cholerae from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986 Jun;153(6):1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- Owen R. L. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology. 1977 Mar;72(3):440–451. [PubMed] [Google Scholar]
- Pappo J., Ermak T. H. Uptake and translocation of fluorescent latex particles by rabbit Peyer's patch follicle epithelium: a quantitative model for M cell uptake. Clin Exp Immunol. 1989 Apr;76(1):144–148. [PMC free article] [PubMed] [Google Scholar]
- Pappo J. Generation and characterization of monoclonal antibodies recognizing follicle epithelial M cells in rabbit gut-associated lymphoid tissues. Cell Immunol. 1989 Apr 15;120(1):31–41. doi: 10.1016/0008-8749(89)90172-x. [DOI] [PubMed] [Google Scholar]
- Pappo J., Owen R. L. Absence of secretory component expression by epithelial cells overlying rabbit gut-associated lymphoid tissue. Gastroenterology. 1988 Nov;95(5):1173–1177. doi: 10.1016/0016-5085(88)90347-2. [DOI] [PubMed] [Google Scholar]
- Pappo J., Steger H. J., Owen R. L. Differential adherence of epithelium overlying gut-associated lymphoid tissue. An ultrastructural study. Lab Invest. 1988 Jun;58(6):692–697. [PubMed] [Google Scholar]
- Roy M. J. Precocious development of lectin (Ulex europaeus agglutinin I) receptors in dome epithelium of gut-associated lymphoid tissues. Cell Tissue Res. 1987 Jun;248(3):483–489. doi: 10.1007/BF00216473. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzin R., Lucia-Jandris P., Michetti P., Fields B. N., Kraehenbuhl J. P., Neutra M. R. Binding and transepithelial transport of immunoglobulins by intestinal M cells: demonstration using monoclonal IgA antibodies against enteric viral proteins. J Cell Biol. 1989 May;108(5):1673–1685. doi: 10.1083/jcb.108.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J. L., Rubin D. H., Finberg R., Kauffman R. S., Sharpe A. H., Trier J. S., Fields B. N. Intestinal M cells: a pathway for entry of reovirus into the host. Science. 1981 Apr 24;212(4493):471–472. doi: 10.1126/science.6259737. [DOI] [PubMed] [Google Scholar]