Abstract
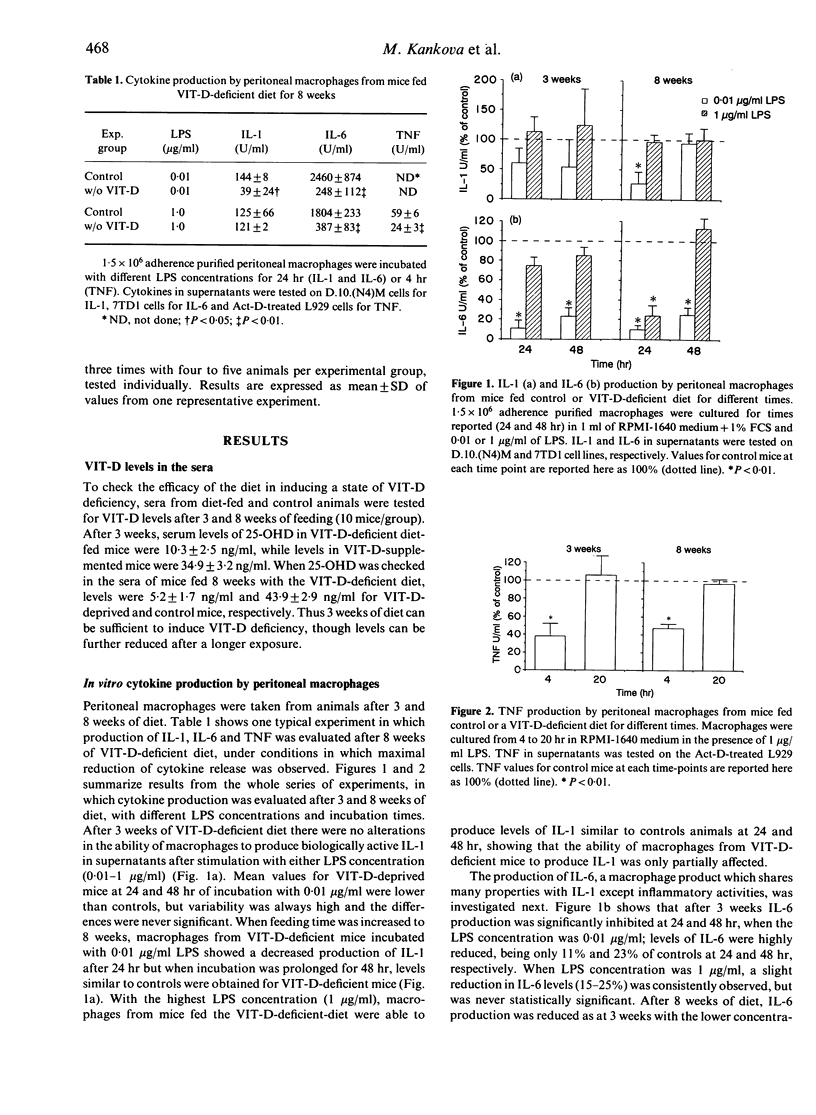
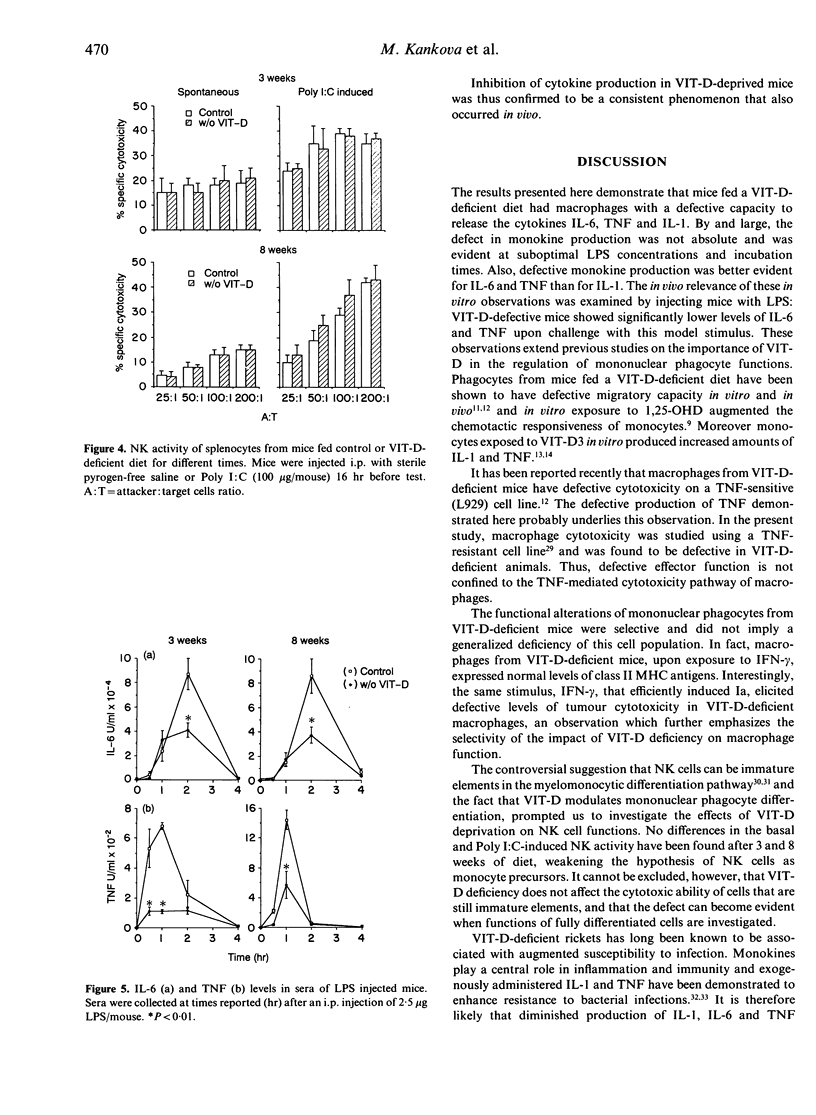
C57Bl/6 female mice fed a Vitamin D (VIT-D)-deficient diet had serum levels of 25-hydroxyvitamin D decreasing with the time of diet exposure (3 and 8 weeks). Cytokine production (IL-6, TNF and IL-1) by peritoneal macrophages cultured in vitro with a standard stimulus, LPS, evaluated in the supernatants as biological activity, was significantly reduced in VIT-D-deficient animals. The defect in monokine production was partial and was evident at suboptimal LPS concentrations and incubation times. I-A antigen expression, induced in macrophages by in vitro exposure to IFN-gamma, was not modified in VIT-D-deficient mice, but IFN-gamma-inducible macrophage cytotoxicity to tumour target cells was significantly decreased in VIT-D-deficient animals. Moreover, basal and Poly I:C-induced NK activity was not modified by VIT-D deficiency. Thus, macrophage functions, such as cytokine production and tumour cytotoxicity induction, are down-modulated in vitro by VIT-D deprivation. To give more support to the relevance of VIT-D availability for cytokine production, TNF and IL-6 have been evaluated in the sera of control and VIT-D-deficient mice given LPS as a model stimulus. Serum peak levels of both cytokines were at least halved in VIT-D-deprived mice. Thus, VIT-D deficiency may represent a model of partial defect of monokine production.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe E., Miyaura C., Sakagami H., Takeda M., Konno K., Yamazaki T., Yoshiki S., Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. S., Sharma O. P., Gacad M. A., Singer F. R. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983 Nov;72(5):1856–1860. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal B. B., Moffat B., Harkins R. N. Human lymphotoxin. Production by a lymphoblastoid cell line, purification, and initial characterization. J Biol Chem. 1984 Jan 10;259(1):686–691. [PubMed] [Google Scholar]
- Baccarini M., Bistoni F., Lohmann-Matthes M. L. Organ-associated macrophage precursor activity: isolation of candidacidal and tumoricidal effectors from the spleens of cyclophosphamide-treated mice. J Immunol. 1986 Feb 1;136(3):837–843. [PubMed] [Google Scholar]
- Bar-Shavit Z., Noff D., Edelstein S., Meyer M., Shibolet S., Goldman R. 1,25-dihydroxyvitamin D3 and the regulation of macrophage function. Calcif Tissue Int. 1981;33(6):673–676. doi: 10.1007/BF02409507. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit Z., Teitelbaum S. L., Reitsma P., Hall A., Pegg L. E., Trial J., Kahn A. J. Induction of monocytic differentiation and bone resorption by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5907–5911. doi: 10.1073/pnas.80.19.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsey R. E., DeLuca H. F., Potts J. T., Jr A rapid assay for 25-OH-vitamin D3 without preparative chromatography. J Clin Endocrinol Metab. 1974 Jun;38(6):1046–1051. doi: 10.1210/jcem-38-6-1046. [DOI] [PubMed] [Google Scholar]
- Boraschi D., Censini S., Tagliabue A. Macrophage antitumor activity: impaired responsiveness to interferon-gamma of macrophages from genetically defective mice. Eur J Immunol. 1984 Nov;14(11):1061–1063. doi: 10.1002/eji.1830141119. [DOI] [PubMed] [Google Scholar]
- Colotta F., Peri G., Villa A., Mantovani A. Rapid killing of actinomycin D-treated tumor cells by human mononuclear cells. I. Effectors belong to the monocyte-macrophage lineage. J Immunol. 1984 Feb;132(2):936–944. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Gavison R., Bar-Shavit Z. Impaired macrophage activation in vitamin D3 deficiency: differential in vitro effects of 1,25-dihydroxyvitamin D3 on mouse peritoneal macrophage functions. J Immunol. 1989 Dec 1;143(11):3686–3690. [PubMed] [Google Scholar]
- Girasole G., Wang J. M., Pedrazzoni M., Pioli G., Balotta C., Passeri M., Lazzarin A., Ridolfo A., Mantovani A. Augmentation of monocyte chemotaxis by 1 alpha,25-dihydroxyvitamin D3. Stimulation of defective migration of AIDS patients. J Immunol. 1990 Oct 15;145(8):2459–2464. [PubMed] [Google Scholar]
- Haddad J. G., Chyu K. J. Competitive protein-binding radioassay for 25-hydroxycholecalciferol. J Clin Endocrinol Metab. 1971 Dec;33(6):992–995. doi: 10.1210/jcem-33-6-992. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., McCain T. A. Basic and clinical concepts related to vitamin D metabolism and action (first of two parts). N Engl J Med. 1977 Nov 3;297(18):974–983. doi: 10.1056/NEJM197711032971804. [DOI] [PubMed] [Google Scholar]
- Hopkins S. J., Humphreys M. Simple, sensitive and specific bioassay of interleukin-1. J Immunol Methods. 1989 Jun 21;120(2):271–276. doi: 10.1016/0022-1759(89)90252-4. [DOI] [PubMed] [Google Scholar]
- Lohmann-Matthes M. L., Domzig W., Roder J. Promonocytes have the functional characteristics of natural killer cells. J Immunol. 1979 Oct;123(4):1883–1886. [PubMed] [Google Scholar]
- Luini W., Boraschi D., Alberti S., Aleotti A., Tagliabue A. Morphological characterization of a cell population responsible for natural killer activity. Immunology. 1981 Aug;43(4):663–668. [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Luini W., Candiani G. P., Spreafico F. Effect of chemotherapeutic agents on natural and BCG-stimulated macrophage cytotoxicity in mice. Int J Immunopharmacol. 1980;2(4):333–339. doi: 10.1016/0192-0561(80)90033-8. [DOI] [PubMed] [Google Scholar]
- Miyaura C., Abe E., Kuribayashi T., Tanaka H., Konno K., Nishii Y., Suda T. 1 alpha,25-Dihydroxyvitamin D3 induces differentiation of human myeloid leukemia cells. Biochem Biophys Res Commun. 1981 Oct 15;102(3):937–943. doi: 10.1016/0006-291x(81)91628-4. [DOI] [PubMed] [Google Scholar]
- Miyaura C., Abe E., Nomura H., Nishii Y., Suda T. 1 alpha,25-Dihydroxyvitamin D3 suppresses proliferation of murine granulocyte-macrophage progenitor cells (CFU-C). Biochem Biophys Res Commun. 1982 Oct 29;108(4):1728–1733. doi: 10.1016/s0006-291x(82)80111-3. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Roth J., Orci L. The vitamin D endocrine system: steroid metabolism, hormone receptors, and biological response (calcium binding proteins). Endocr Rev. 1982 Fall;3(4):331–366. doi: 10.1210/edrv-3-4-331. [DOI] [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Peri G., Rossi V., Taraboletti G., Erroi A., Mantovani A. Ia antigen expression and IL-1 activity in murine tumour-associated macrophages. Immunology. 1986 Dec;59(4):527–533. [PMC free article] [PubMed] [Google Scholar]
- Provvedini D. M., Deftos L. J., Manolagas S. C. 1,25-Dihydroxyvitamin D3 promotes in vitro morphologic and enzymatic changes in normal human monocytes consistent with their differentiation into macrophages. Bone. 1986;7(1):23–28. doi: 10.1016/8756-3282(86)90148-1. [DOI] [PubMed] [Google Scholar]
- Provvedini D. M., Tsoukas C. D., Deftos L. J., Manolagas S. C. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983 Sep 16;221(4616):1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- Rigby W. F., Noelle R. J., Krause K., Fanger M. W. The effects of 1,25-dihydroxyvitamin D3 on human T lymphocyte activation and proliferation: a cell cycle analysis. J Immunol. 1985 Oct;135(4):2279–2286. [PubMed] [Google Scholar]
- Rook G. A., Taverne J., Leveton C., Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987 Oct;62(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Sironi M., Breviario F., Proserpio P., Biondi A., Vecchi A., Van Damme J., Dejana E., Mantovani A. IL-1 stimulates IL-6 production in endothelial cells. J Immunol. 1989 Jan 15;142(2):549–553. [PubMed] [Google Scholar]
- Ströder J., Kasal P. Evaluation of phagocytosis in rickets. Acta Paediatr Scand. 1970 May;59(3):288–292. doi: 10.1111/j.1651-2227.1970.tb09005.x. [DOI] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- van der Meer J. W., Barza M., Wolff S. M., Dinarello C. A. A low dose of recombinant interleukin 1 protects granulocytopenic mice from lethal gram-negative infection. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1620–1623. doi: 10.1073/pnas.85.5.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J. W. The effects of recombinant interleukin-1 and recombinant tumor necrosis factor on non-specific resistance to infection. Biotherapy. 1988;1(1):19–25. doi: 10.1007/BF02170132. [DOI] [PubMed] [Google Scholar]


