Abstract
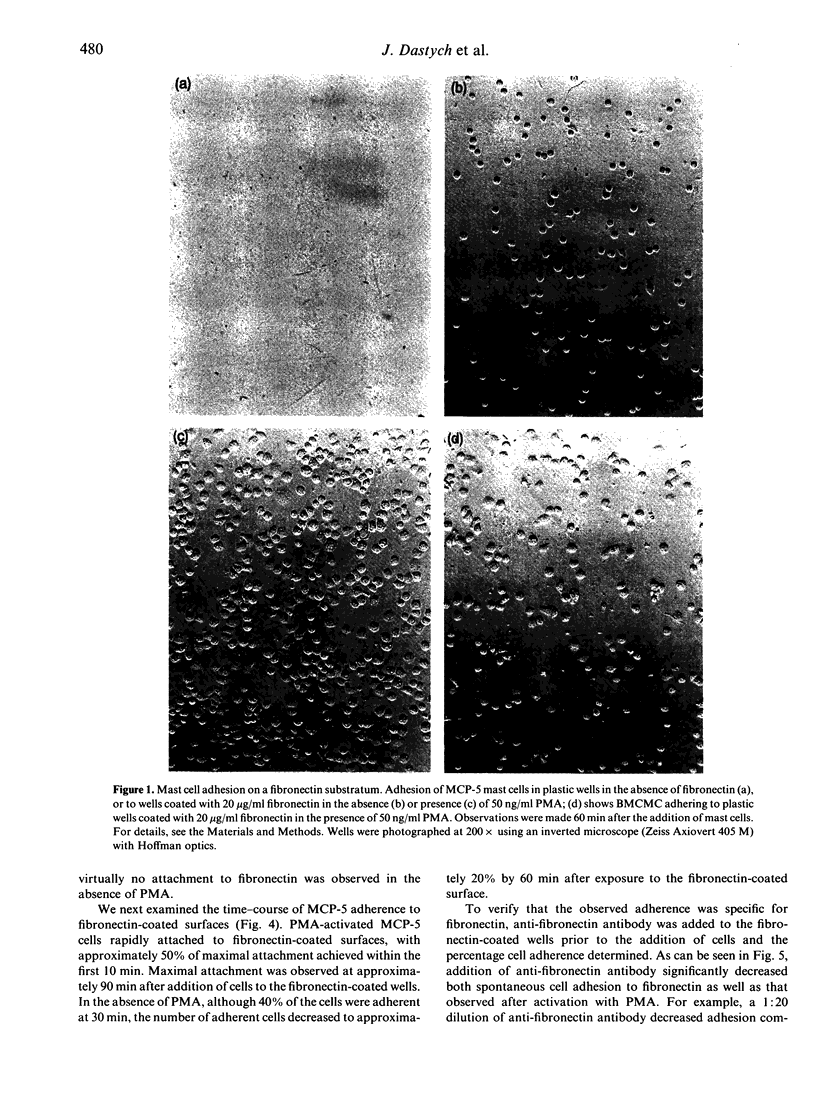
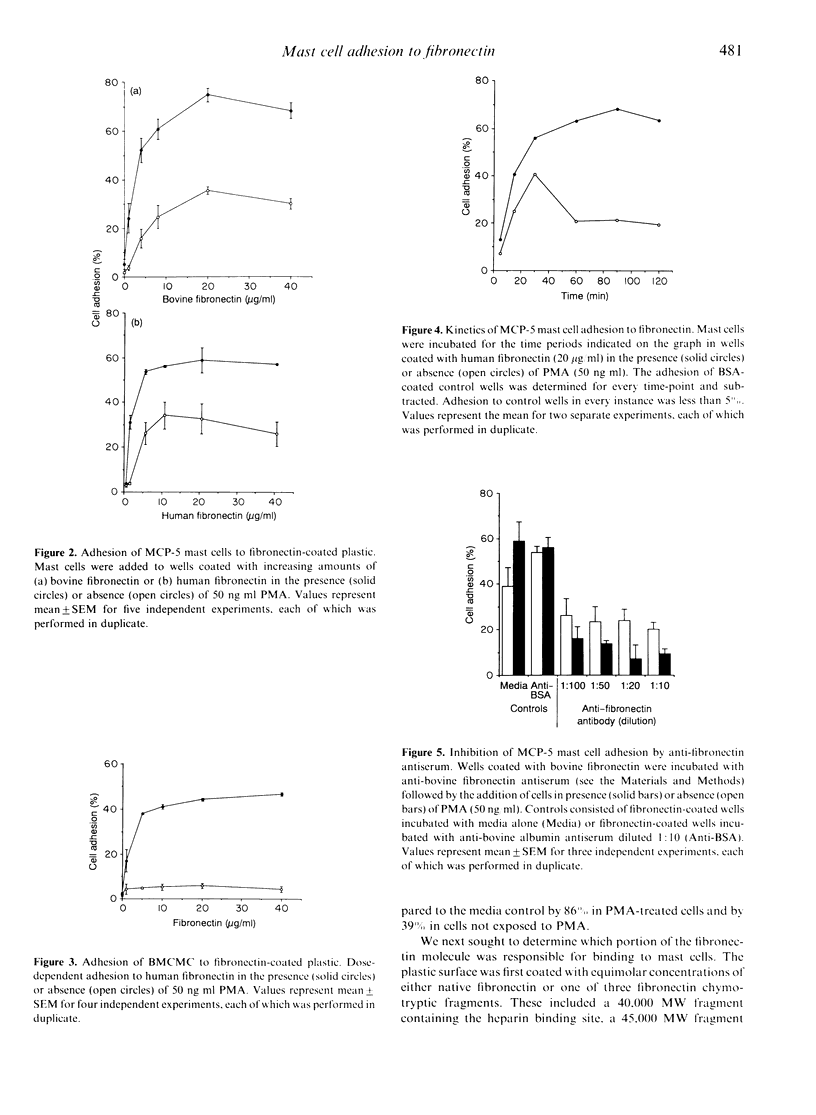
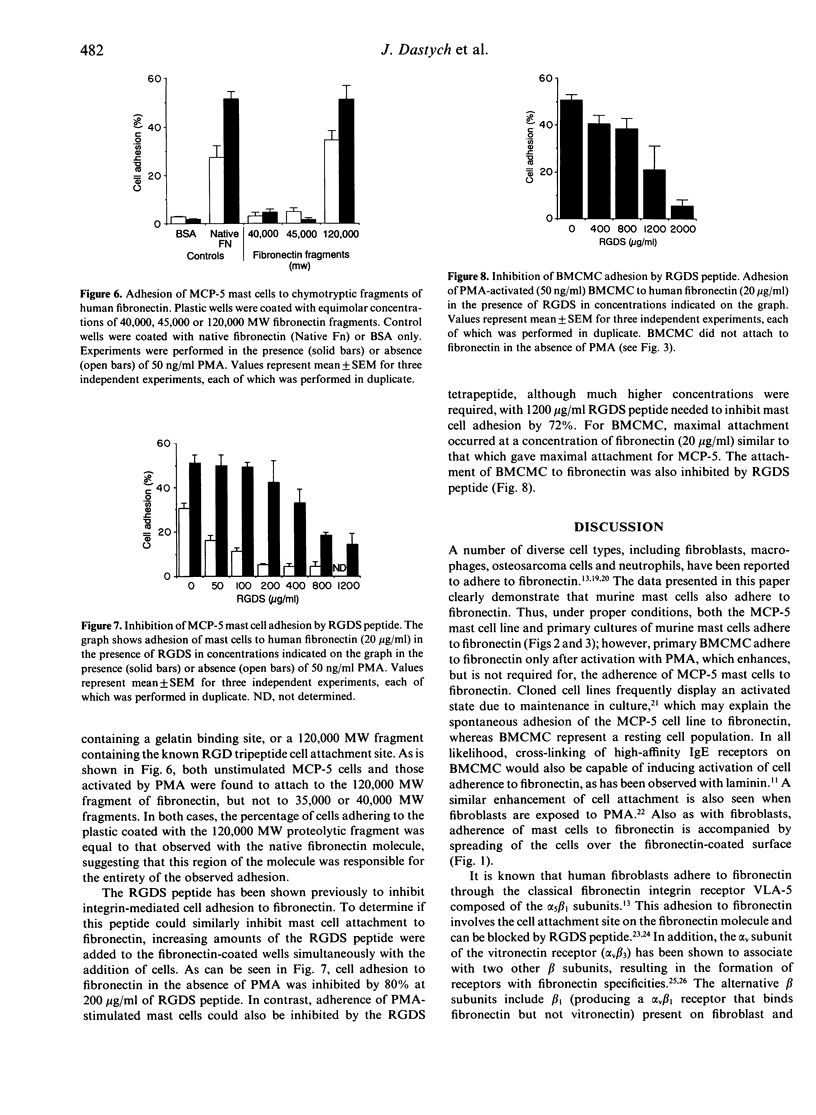
The MCP-5 murine mast cell line, as well as primary bone marrow-derived cultured mast cells (BMCMC), are demonstrated to bind to fibronectin, a ubiquitous adhesion protein of the extracellular matrix. BMCMC required activation by phorbol myristate acetate (PMA) to adhere to fibronectin, whereas MCP-5 displayed spontaneous adherence. The binding of both MCP-5 and BMCMC was dose dependent, with maximal adhesion at a fibronectin concentration of 20 micrograms/ml. The 120,000 molecular weight (MW) proteolytic fragment of fibronectin containing the RGDS cell attachment site was able to substitute for the native fibronectin molecule in promoting mast cell attachment. Mast cell adhesion to fibronectin, in addition, could be inhibited by the RGDS peptide alone. These data suggest that, in addition to the previously described mast cell-laminin interactions, mast cells also adhere to fibronectin, thus providing further insight into their tissue localization and possible roles in processes such as wound healing and fibrosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Watt F. M. Fibronectin inhibits the terminal differentiation of human keratinocytes. Nature. 1989 Jul 27;340(6231):307–309. doi: 10.1038/340307a0. [DOI] [PubMed] [Google Scholar]
- Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989 Aug;109(2):863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Amrani D., Mosesson M. W., Bianco C. Receptors for cold-insoluble globulin (plasma fibronectin) on human monocytes. J Exp Med. 1981 Jan 1;153(1):42–60. doi: 10.1084/jem.153.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. J. Phorbol ester stimulation of fibronectin-mediated cell adhesion. Biochem Biophys Res Commun. 1988 Sep 15;155(2):603–607. doi: 10.1016/s0006-291x(88)80537-0. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Smith J. W., Cooper H. M., Quaranta V. A novel vitronectin receptor integrin (alpha v beta x) is responsible for distinct adhesive properties of carcinoma cells. Cell. 1989 Apr 7;57(1):59–69. doi: 10.1016/0092-8674(89)90172-4. [DOI] [PubMed] [Google Scholar]
- Curtis A. S., Forrester J. V., McInnes C., Lawrie F. Adhesion of cells to polystyrene surfaces. J Cell Biol. 1983 Nov;97(5 Pt 1):1500–1506. doi: 10.1083/jcb.97.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Comoglio P. M., Tarone G. A 135,000 molecular weight plasma membrane glycoprotein involved in fibronectin-mediated cell adhesion. Immunofluorescence localization in normal and RSV-transformed fibroblasts. Exp Cell Res. 1986 Mar;163(1):47–62. doi: 10.1016/0014-4827(86)90557-4. [DOI] [PubMed] [Google Scholar]
- Hedin U., Bottger B. A., Luthman J., Johansson S., Thyberg J. A substrate of the cell-attachment sequence of fibronectin (Arg-Gly-Asp-Ser) is sufficient to promote transition of arterial smooth muscle cells from a contractile to a synthetic phenotype. Dev Biol. 1989 Jun;133(2):489–501. doi: 10.1016/0012-1606(89)90052-3. [DOI] [PubMed] [Google Scholar]
- Holers V. M., Ruff T. G., Parks D. L., McDonald J. A., Ballard L. L., Brown E. J. Molecular cloning of a murine fibronectin receptor and its expression during inflammation. Expression of VLA-5 is increased in activated peritoneal macrophages in a manner discordant from major histocompatibility complex class II. J Exp Med. 1989 May 1;169(5):1589–1605. doi: 10.1084/jem.169.5.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanir N., Ciano P. S., Van de Water L., McDonagh J., Dvorak A. M., Dvorak H. F. Macrophage migration in fibrin gel matrices. II. Effects of clotting factor XIII, fibronectin, and glycosaminoglycan content on cell migration. J Immunol. 1988 Apr 1;140(7):2340–2349. [PubMed] [Google Scholar]
- Lewandowska K., Balachander N., Sukenik C. N., Culp L. A. Modulation of fibronectin adhesive functions for fibroblasts and neural cells by chemically derivatized substrata. J Cell Physiol. 1989 Nov;141(2):334–345. doi: 10.1002/jcp.1041410215. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Mercurio A. M., Shaw L. M. Macrophage interactions with laminin: PMA selectively induces the adherence and spreading of mouse macrophages on a laminin substratum. J Cell Biol. 1988 Nov;107(5):1873–1880. doi: 10.1083/jcb.107.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Srimal S., Farber C., Sanchez E., Kabbash L., Asch A., Gailit J., Wright S. D. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins. J Cell Biol. 1989 Sep;109(3):1341–1349. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris D. A., Clark R. A., Swigart L. M., Huff J. C., Weston W. L., Howell S. E. Fibronectin fragment(s) are chemotactic for human peripheral blood monocytes. J Immunol. 1982 Oct;129(4):1612–1618. [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Hayman E. G., Ruoslahti E. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981 Oct;26(2 Pt 2):259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Thiery J. P., Duband J. L., Tucker G. C. Cell migration in the vertebrate embryo: role of cell adhesion and tissue environment in pattern formation. Annu Rev Cell Biol. 1985;1:91–113. doi: 10.1146/annurev.cb.01.110185.000515. [DOI] [PubMed] [Google Scholar]
- Thompson H. L., Burbelo P. D., Metcalfe D. D. Regulation of adhesion of mouse bone marrow-derived mast cells to laminin. J Immunol. 1990 Nov 15;145(10):3425–3431. [PubMed] [Google Scholar]
- Thompson H. L., Burbelo P. D., Segui-Real B., Yamada Y., Metcalfe D. D. Laminin promotes mast cell attachment. J Immunol. 1989 Oct 1;143(7):2323–2327. [PubMed] [Google Scholar]
- Vogel B. E., Tarone G., Giancotti F. G., Gailit J., Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition (alpha v beta 1). J Biol Chem. 1990 Apr 15;265(11):5934–5937. [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G., Piotrowicz R. S., Kunicki T. J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988 Nov;107(5):1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]



