Abstract
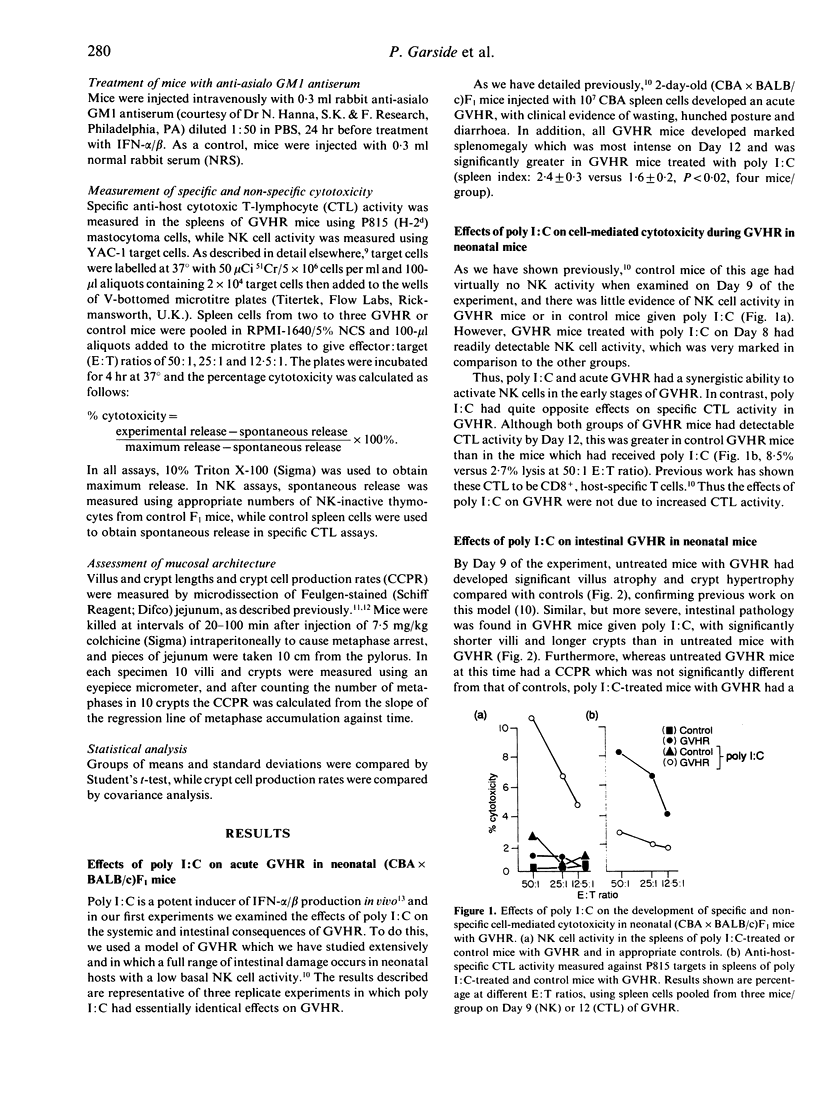
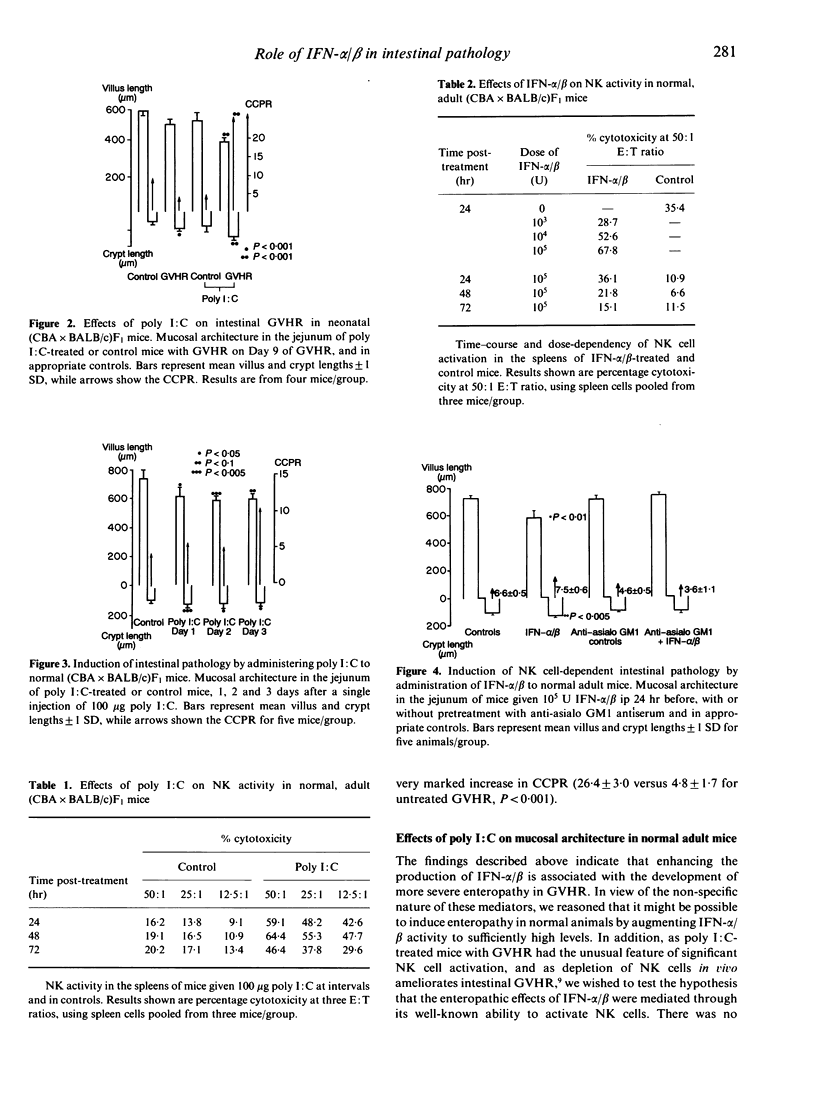
We have investigated the role of interferon-alpha/beta (IFN-alpha/beta) and IFN-dependent effector cells in causing enteropathy in mice. The IFN-inducer polyinosinic:polycytydylic acid (poly I:C) augmented the natural killer (NK) cell activation normally seen in neonatal (CBA x BALB/c)F1 mice with graft-versus-host reaction (GVHR) and exacerbated the systemic and intestinal consequences of GVHR. Poly I:C itself produced a similar pattern of intestinal pathology when administered to normal mice. The effects of poly I:C on NK cell activity and intestinal architecture in normal mice could be reproduced by a single injection of purified IFN-alpha/beta and the intestinal lesions caused by IFN-alpha/beta were prevented by in vivo depletion of NK cells with anti-asialo GM1. These results indicate that IFN-alpha/beta may play an important role in immunologically mediated enteropathies by virtue of its ability to activate NK cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borland A., Mowat A. M., Parrott D. M. Augmentation of intestinal and peripheral natural killer cell activity during the graft-versus-host reaction in mice. Transplantation. 1983 Nov;36(5):513–519. doi: 10.1097/00007890-198311000-00009. [DOI] [PubMed] [Google Scholar]
- Bril H., Benner R. Graft-vs.-host reactions: mechanisms and contemporary theories. Crit Rev Clin Lab Sci. 1985;22(1):43–95. doi: 10.1080/10408368509176815. [DOI] [PubMed] [Google Scholar]
- Carman P. S., Ernst P. B., Rosenthal K. L., Clark D. A., Befus A. D., Bienenstock J. Intraepithelial leukocytes contain a unique subpopulation of NK-like cytotoxic cells active in the defense of gut epithelium to enteric murine coronavirus. J Immunol. 1986 Mar 1;136(5):1548–1553. [PubMed] [Google Scholar]
- Charley M. R., Mikhael A., Bennett M., Gilliam J. N., Sontheimer R. D. Prevention of lethal, minor-determinate graft-host disease in mice by the in vivo administration of anti-asialo GM1. J Immunol. 1983 Nov;131(5):2101–2103. [PubMed] [Google Scholar]
- Cleveland M. G., Annable C. R., Klimpel G. R. In vivo and in vitro production of IFN-beta and IFN-gamma during graft vs host disease. J Immunol. 1988 Nov 15;141(10):3349–3356. [PubMed] [Google Scholar]
- Cleveland M. G., Lane R. G., Klimpel G. R. Enhanced interferon-alpha/beta (IFN-alpha/beta) and defective IFN-gamma production in chronic graft versus host disease: a potential mechanism for immunosuppression. Cell Immunol. 1987 Nov;110(1):120–130. doi: 10.1016/0008-8749(87)90106-7. [DOI] [PubMed] [Google Scholar]
- Degliantoni G., Murphy M., Kobayashi M., Francis M. K., Perussia B., Trinchieri G. Natural killer (NK) cell-derived hematopoietic colony-inhibiting activity and NK cytotoxic factor. Relationship with tumor necrosis factor and synergism with immune interferon. J Exp Med. 1985 Nov 1;162(5):1512–1530. doi: 10.1084/jem.162.5.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeu J. Y., Heinbaugh J. A., Holden H. T., Herberman R. B. Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J Immunol. 1979 Jan;122(1):175–181. [PubMed] [Google Scholar]
- Felstein M. V., Mowat A. M. Experimental studies of immunologically mediated enteropathy: IV. Correlation between immune effector mechanisms and type of enteropathy during a GvHR in neonatal mice of different ages. Clin Exp Immunol. 1988 Apr;72(1):108–112. [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T., Ferguson A. Hypersensitivity reactions in the small intestine. III. The effects of allograft rejection and of graft-versus-host disease on epithelial cell kinetics. Cell Tissue Kinet. 1977 Jul;10(4):301–312. [PubMed] [Google Scholar]
- MacDonald T. T., Hutchings P., Choy M. Y., Murch S., Cooke A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol. 1990 Aug;81(2):301–305. doi: 10.1111/j.1365-2249.1990.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M. Antibodies to IFN-gamma prevent immunologically mediated intestinal damage in murine graft-versus-host reaction. Immunology. 1989 Sep;68(1):18–23. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Felstein M. V. Experimental studies of immunologically mediated enteropathy. II. Role of natural killer cells in the intestinal phase of murine graft-versus-host reaction. Immunology. 1987 Jun;61(2):179–183. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Felstein M. V. Experimental studies of immunologically mediated enteropathy. V. Destructive enteropathy during an acute graft-versus-host reaction in adult BDF1 mice. Clin Exp Immunol. 1990 Feb;79(2):279–284. doi: 10.1111/j.1365-2249.1990.tb05191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Ferguson A. Hypersensitivity reactions in the small intestine. 6. Pathogenesis of the graft-versus-host reaction in the small intestinal mucosa of the mouse. Transplantation. 1981 Sep;32(3):238–243. doi: 10.1097/00007890-198109000-00011. [DOI] [PubMed] [Google Scholar]
- Peres A., Amlani S., Kornbluth M., Seemayer T. A., Lapp W. S. The effects of polyinosinic:polycytidylic acid on the graft-versus-host reaction. III: Increased severity of the reaction with delayed pI:C treatment. Transplantation. 1989 Jul;48(1):80–84. [PubMed] [Google Scholar]
- Peres A., Nestel F. P., Seemayer T. A., Lapp W. S. The effects of polyinosinic:polycytidylic acid (pI:C) on the graft-vs-host (GVH) reaction. II. Increased NK-mediated rejection on C57BL/6 lymphocytes by (C57BL/6 X A)F1 mice. J Immunol. 1986 Dec 1;137(11):3420–3427. [PubMed] [Google Scholar]
- Piguet P. F., Grau G. E., Allet B., Vassalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs.-host disease. J Exp Med. 1987 Nov 1;166(5):1280–1289. doi: 10.1084/jem.166.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varkila K. Depletion of asialo-GM1+ cells from the F1 recipient mice prior to irradiation and transfusion of parental spleen cells prevents mortality to acute graft-versus-host disease and induction of anti-host specific cytotoxic T cells. Clin Exp Immunol. 1987 Sep;69(3):652–659. [PMC free article] [PubMed] [Google Scholar]


