Abstract
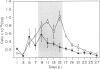
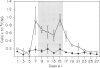
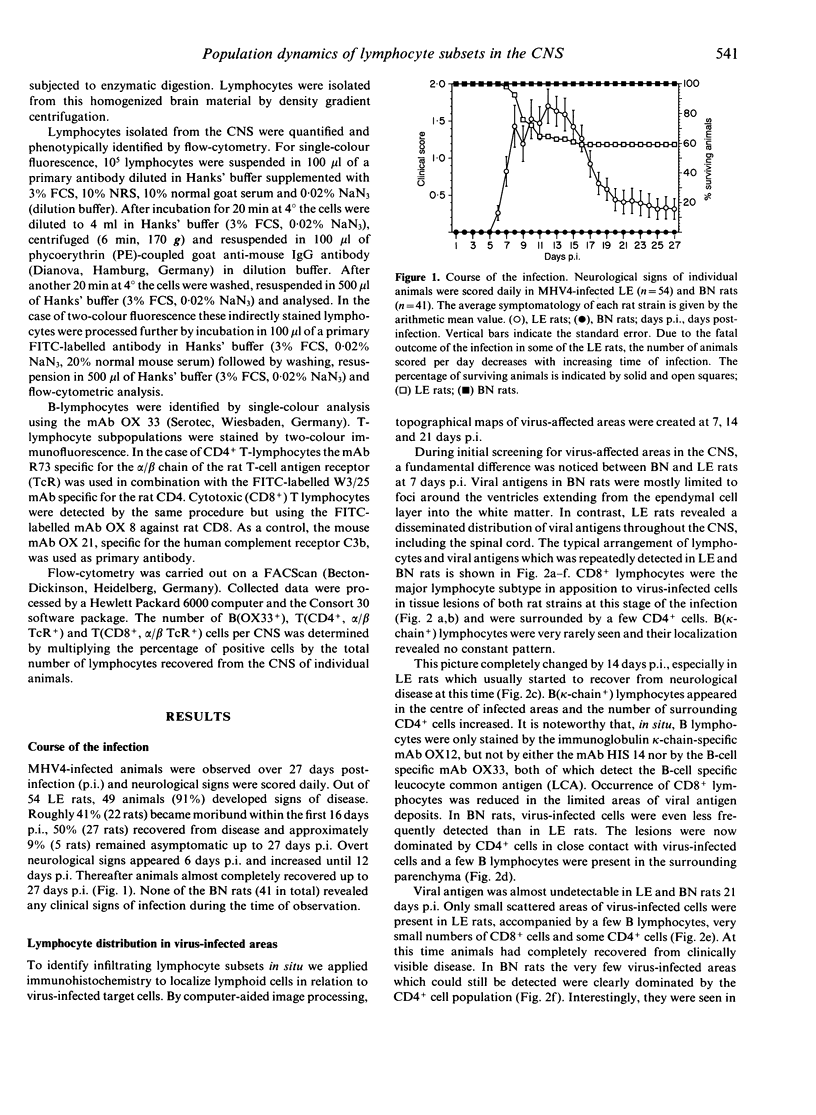
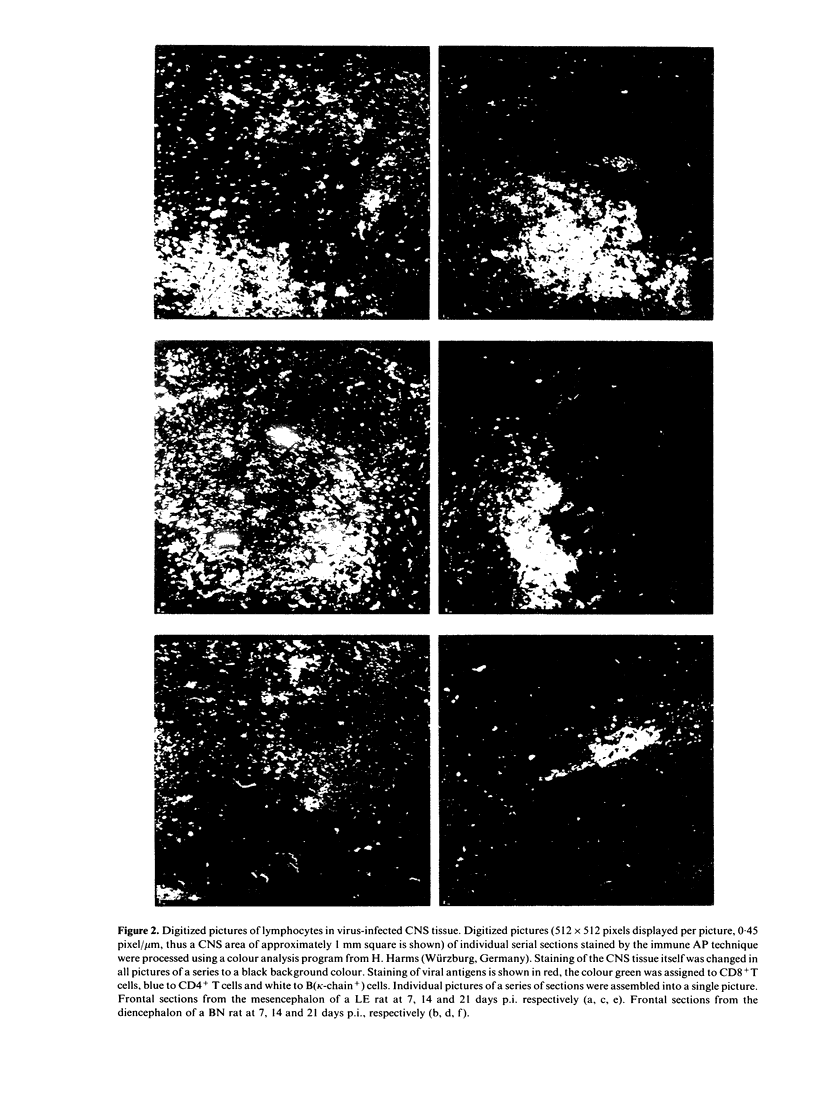
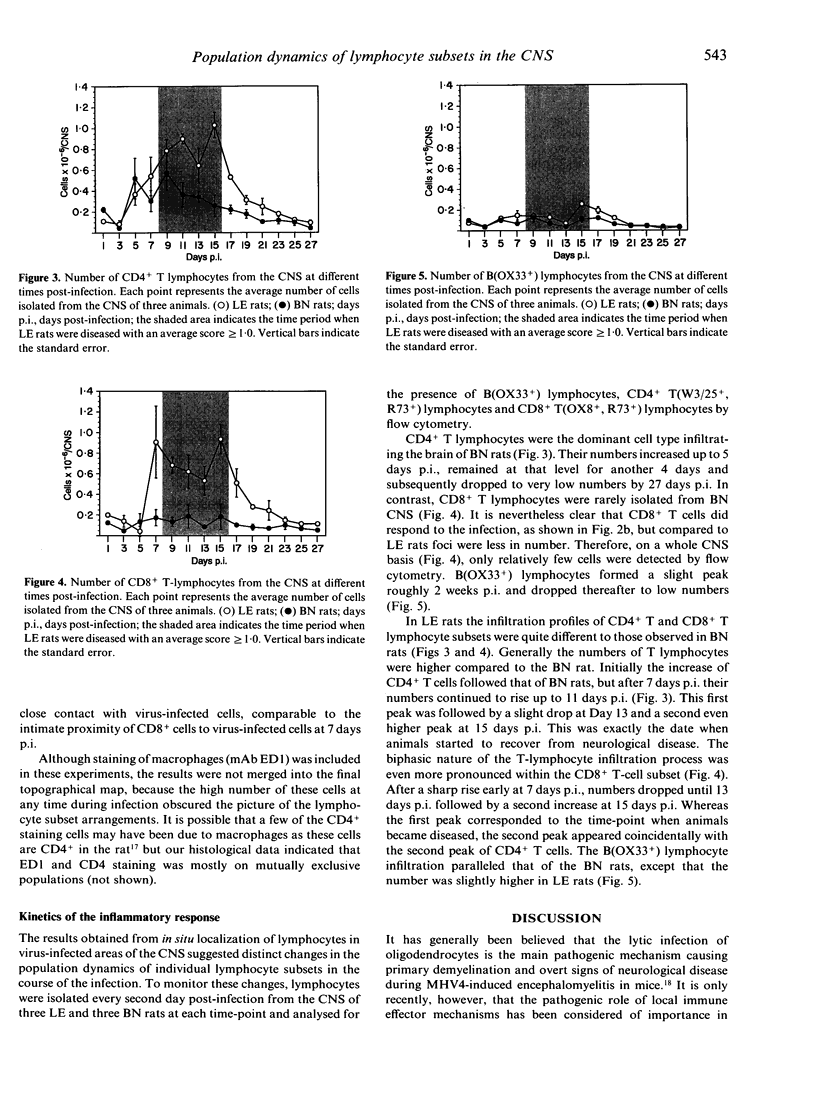
The inflammatory response in the central nervous system (CNS) of rats with differing susceptibility to demyelinating encephalitis induced by coronavirus MHV4 was characterized. Topographical maps showing the arrangement of infiltrating lymphocyte subsets in virus-infected tissue were developed by digital-image processing of immunohistologically stained CNS sections. The kinetics of the inflammatory process was evaluated by flow-cytometry on lymphocytes isolated from the CNS. Cumulative data obtained with these two techniques demonstrated the following features. In susceptible Lewis (LE) rats, viral antigens were disseminated throughout the CNS, including spinal cord. Onset as well as recovery from neurological disease was associated with a steep rise of infiltrating CD8+ T cells, which localized in close contact to virus-infected cells. Accompanying convalescence was a slight increase in B(OX33+) cells in the CNS and the accumulation of immunoglobulin-containing cells in the centre of virus-infected areas. In clinically resistant Brown Norway (BN) rats, virus-infected cells were mainly restricted to small periventricular foci and the extent of lymphocyte infiltration was never as high as that found at any time during the course of infection in LE rats. There were striking differences in the CD8+ T-cell population compared to LE rats. Cells of this phenotype were identified in virus-affected areas of BN rats only early after infection, and their infiltration profile revealed much lower quantities than in the CNS of susceptible LE rats. Although the population dynamics of B(OX33+) lymphocytes were comparable in BN and LE rats, as determined by flow-cytometry, less immunoglobulin-containing B cells were detected in virus-infected areas of BN rats.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Allan J. E., Lynch F., Ceredig R. Dissection of an inflammatory process induced by CD8+ T cells. Immunol Today. 1990 Feb;11(2):55–59. doi: 10.1016/0167-5699(90)90019-6. [DOI] [PubMed] [Google Scholar]
- Dörries R., Watanabe R., Wege H., ter Meulen V. Analysis of the intrathecal humoral immune response in Brown Norway (BN) rats, infected with the murine coronavirus JHM. J Neuroimmunol. 1987 Apr;14(3):305–316. doi: 10.1016/0165-5728(87)90017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörries R., Watanabe R., Wege H., ter Meulen V. Murine coronavirus-induced encephalomyelitis in rats: analysis of immunoglobulins and virus-specific antibodies in serum and cerebrospinal fluid. J Neuroimmunol. 1986 Aug;12(2):131–142. doi: 10.1016/0165-5728(86)90026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung L., Barclay A. N., Brandon M. R., Sim E., Porter R. R. Purification of human C3b inactivator by monoclonal-antibody affinity chromatography. Biochem J. 1982 Apr 1;203(1):293–298. doi: 10.1042/bj2030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S. V., Fowler M. H. A repopulation assay for B and T lymphocyte stem cells employing radiation chimaeras. Cell Tissue Kinet. 1981 Jul;14(4):445–464. doi: 10.1111/j.1365-2184.1981.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Hünig T., Wallny H. J., Hartley J. K., Lawetzky A., Tiefenthaler G. A monoclonal antibody to a constant determinant of the rat T cell antigen receptor that induces T cell activation. Differential reactivity with subsets of immature and mature T lymphocytes. J Exp Med. 1989 Jan 1;169(1):73–86. doi: 10.1084/jem.169.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroese F. G., Wubbena A. S., Opstelten D., Deenen G. J., Schwander E. H., De Leij L., Vos H., Poppema S., Volberda J., Nieuwenhuis P. B lymphocyte differentiation in the rat: production and characterization of monoclonal antibodies to B lineage-associated antigens. Eur J Immunol. 1987 Jul;17(7):921–928. doi: 10.1002/eji.1830170705. [DOI] [PubMed] [Google Scholar]
- Lawetzky A., Tiefenthaler G., Kubo R., Hünig T. Identification and characterization of rat T cell subpopulations expressing T cell receptors alpha/beta and gamma/delta. Eur J Immunol. 1990 Feb;20(2):343–349. doi: 10.1002/eji.1830200217. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Paul W. E., Ohara J. B-cell stimulatory factor-1/interleukin 4. Annu Rev Immunol. 1987;5:429–459. doi: 10.1146/annurev.iy.05.040187.002241. [DOI] [PubMed] [Google Scholar]
- Schwender S., Imrich H., Dörries R. The pathogenic role of virus-specific antibody-secreting cells in the central nervous system of rats with different susceptibility to coronavirus-induced demyelinating encephalitis. Immunology. 1991 Nov;74(3):533–538. [PMC free article] [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. Suppression of IgE responses in inbred rats by repeated respiratory tract exposure to antigen: responder phenotype influences isotype specificity of induced tolerance. Eur J Immunol. 1984 Oct;14(10):893–897. doi: 10.1002/eji.1830141006. [DOI] [PubMed] [Google Scholar]
- Wang F. I., Stohlman S. A., Fleming J. O. Demyelination induced by murine hepatitis virus JHM strain (MHV-4) is immunologically mediated. J Neuroimmunol. 1990 Nov;30(1):31–41. doi: 10.1016/0165-5728(90)90050-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R., Wege H., ter Meulen V. Adoptive transfer of EAE-like lesions from rats with coronavirus-induced demyelinating encephalomyelitis. Nature. 1983 Sep 8;305(5930):150–153. doi: 10.1038/305150a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R., Wege H., ter Meulen V. Comparative analysis of coronavirus JHM-induced demyelinating encephalomyelitis in Lewis and Brown Norway rats. Lab Invest. 1987 Oct;57(4):375–384. [PubMed] [Google Scholar]
- Wege H., Müller A., ter Meulen V. Genomic RNA of the murine coronavirus JHM. J Gen Virol. 1978 Nov;41(2):217–227. doi: 10.1099/0022-1317-41-2-217. [DOI] [PubMed] [Google Scholar]
- Weiner L. P. Pathogenesis of demyelination induced by a mouse hepatitis. Arch Neurol. 1973 May;28(5):298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Williamson J. S., Stohlman S. A. Effective clearance of mouse hepatitis virus from the central nervous system requires both CD4+ and CD8+ T cells. J Virol. 1990 Sep;64(9):4589–4592. doi: 10.1128/jvi.64.9.4589-4592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]