Abstract
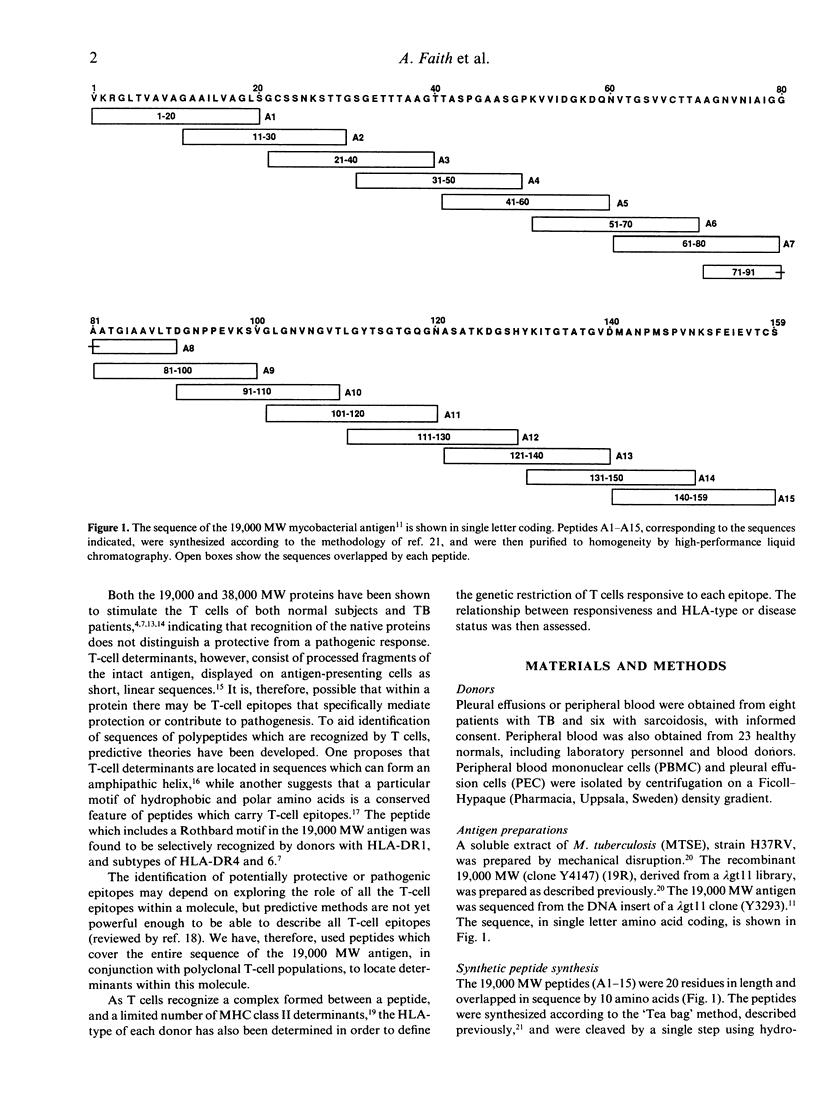
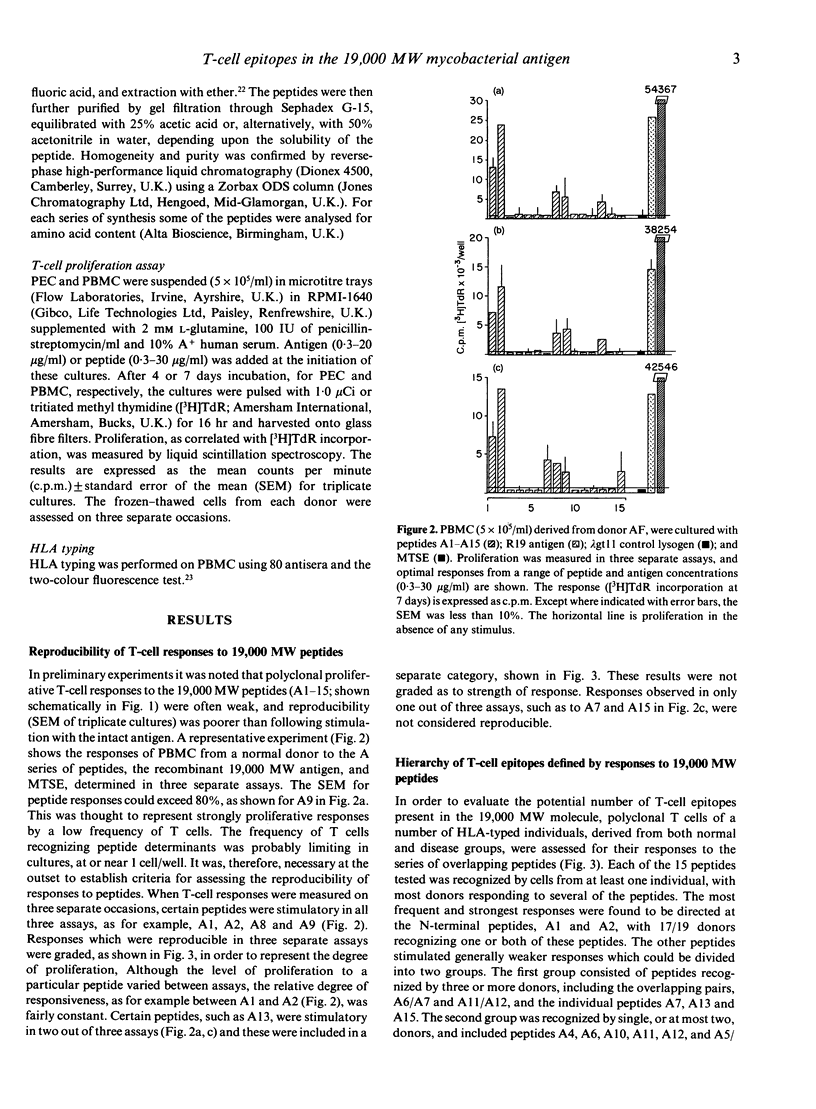
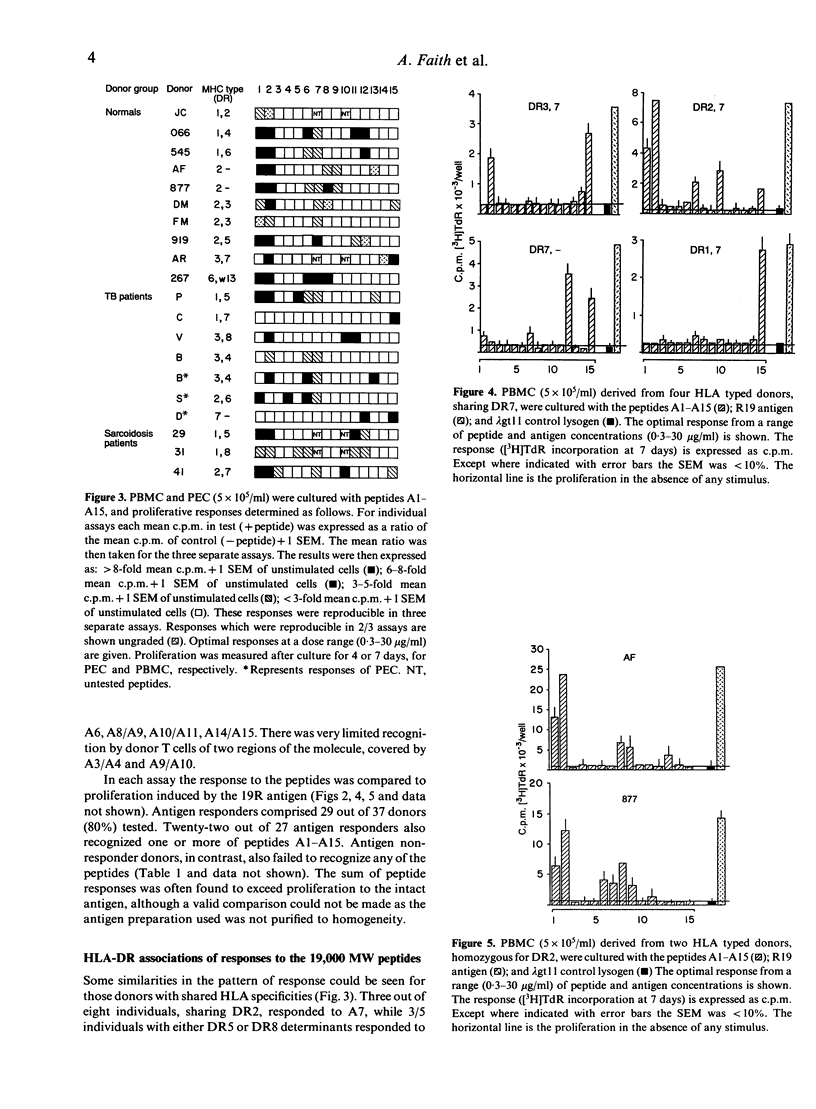
The potential number of T-cell epitopes in the 19,000 molecular weight (MW) antigen has been investigated using overlapping peptides which comprise the complete sequence. Sixteen potential epitopes could be deduced from the responses to these peptides by polyclonal T cells derived from 22 antigen-responsive donors. The majority of epitopes were not predicted by either of the major paradigms, the Rothbard motif and the amphipathic helix. A hierarchy of epitopes was indicated by the responses, which ranged from strong and frequent in the N-terminal region, to moderate or weak elsewhere. Some epitopes were restricted by single HLA-DR determinants, or families of determinants sharing structural features in common, whilst the two N-terminal peptides were recognized by donors with a diversity of DR types. The high degree of T-cell recognition of the N-terminal region may be of relevance to the design of a sub-unit vaccine capable of priming T cells against Mycobacterium tuberculosis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. B., Hansen E. B. Structure and mapping of antigenic domains of protein antigen b, a 38,000-molecular-weight protein of Mycobacterium tuberculosis. Infect Immun. 1989 Aug;57(8):2481–2488. doi: 10.1128/iai.57.8.2481-2488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbridge K. R., Booth R. J., Watson J. D., Lathigra R. B. Nucleotide sequence of the 19 kDa antigen gene from Mycobacterium tuberculosis. Nucleic Acids Res. 1989 Feb 11;17(3):1249–1249. doi: 10.1093/nar/17.3.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzofsky J. A., Bensussan A., Cease K. B., Bourge J. F., Cheynier R., Lurhuma Z., Salaün J. J., Gallo R. C., Shearer G. M., Zagury D. Antigenic peptides recognized by T lymphocytes from AIDS viral envelope-immune humans. Nature. 1988 Aug 25;334(6184):706–708. doi: 10.1038/334706a0. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Cease K. B., Berzofsky J. A. Influences of antigen processing on the expression of the T cell repertoire. Evidence for MHC-specific hindering structures on the products of processing. J Exp Med. 1988 Jul 1;168(1):357–373. doi: 10.1084/jem.168.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Basten A., Inglis A. S. Immunoreactivity of a 70 kD protein purified from Mycobacterium bovis Bacillus Calmette-Guerin by monoclonal antibody affinity chromatography. J Exp Med. 1986 Sep 1;164(3):695–708. doi: 10.1084/jem.164.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Cease K. B., Berkower I., York-Jolley J., Berzofsky J. A. T cell clones specific for an amphipathic alpha-helical region of sperm whale myoglobin show differing fine specificities for synthetic peptides. A multiview/single structure interpretation of immunodominance. J Exp Med. 1986 Nov 1;164(5):1779–1784. doi: 10.1084/jem.164.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi C., Berzofsky J. A. T-cell antigenic sites tend to be amphipathic structures. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7048–7052. doi: 10.1073/pnas.82.20.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. P., Bäckström B. T., Booth R. J., Love S. G., Harding D. R., Watson J. D. The mapping of epitopes of the 18-kDa protein of Mycobacterium leprae recognized by murine T cells in a proliferation assay. J Immunol. 1989 Sep 15;143(6):2006–2012. [PubMed] [Google Scholar]
- Houghten R. A., Bray M. K., Degraw S. T., Kirby C. J. Simplified procedure for carrying out simultaneous multiple hydrogen fluoride cleavages of protected peptide resins. Int J Pept Protein Res. 1986 Jun;27(6):673–678. doi: 10.1111/j.1399-3011.1986.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Kilgus J., Romagnoli P., Guttinger M., Stuber D., Adorini L., Sinigaglia F. Vaccine T-cell epitope selection by a peptide competition assay. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1629–1633. doi: 10.1073/pnas.86.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Ivanyi J., Rees A. D., Rothbard J. B., Howland K., Young R. A., Young D. B. Mapping of T cell epitopes using recombinant antigens and synthetic peptides. EMBO J. 1987 May;6(5):1245–1249. doi: 10.1002/j.1460-2075.1987.tb02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Rees A. D., Bal V., Ikeda H., Wilkinson D., De Vries R. R., Rothbard J. B. Prediction and identification of an HLA-DR-restricted T cell determinant in the 19-kDa protein of Mycobacterium tuberculosis. Eur J Immunol. 1988 Jun;18(6):973–976. doi: 10.1002/eji.1830180623. [DOI] [PubMed] [Google Scholar]
- Lehmann P. V., Cardinaux F., Appella E., Muller S., Falcioni F., Adorini L., Nagy Z. A. Inhibition of T cell response with peptides is influenced by both peptide-binding specificity of major histocompatibility complex molecules and susceptibility of T cells to blocking. Eur J Immunol. 1989 Jun;19(6):1071–1077. doi: 10.1002/eji.1830190617. [DOI] [PubMed] [Google Scholar]
- Lombardi G., Sidhu S., Batchelor J. R., Lechler R. I. Allorecognition of DR1 by T cells from a DR4/DRw13 responder mimics self-restricted recognition of endogenous peptides. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4190–4194. doi: 10.1073/pnas.86.11.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R. Synthetic T and B cell recognition sites: implications for vaccine development. Adv Immunol. 1989;45:195–282. doi: 10.1016/s0065-2776(08)60694-x. [DOI] [PubMed] [Google Scholar]
- Munk M. E., Schoel B., Kaufmann S. H. T cell responses of normal individuals towards recombinant protein antigens of Mycobacterium tuberculosis. Eur J Immunol. 1988 Nov;18(11):1835–1838. doi: 10.1002/eji.1830181128. [DOI] [PubMed] [Google Scholar]
- Oftung F., Mustafa A. S., Husson R., Young R. A., Godal T. Human T cell clones recognize two abundant Mycobacterium tuberculosis protein antigens expressed in Escherichia coli. J Immunol. 1987 Feb 1;138(3):927–931. [PubMed] [Google Scholar]
- Oftung F., Mustafa A. S., Shinnick T. M., Houghten R. A., Kvalheim G., Degre M., Lundin K. E., Godal T. Epitopes of the Mycobacterium tuberculosis 65-kilodalton protein antigen as recognized by human T cells. J Immunol. 1988 Oct 15;141(8):2749–2754. [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Rees A. D., Scoging A., Dobson N., Praputpittaya K., Young D., Ivanyi J., Lamb J. R. T cell activation by anti-idiotypic antibody: mechanism of interaction with antigen-reactive T cells. Eur J Immunol. 1987 Feb;17(2):197–201. doi: 10.1002/eji.1830170208. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Adorini L., Colon S. M., Buus S., Grey H. M. Capacity of intact proteins to bind to MHC class II molecules. J Immunol. 1989 Aug 15;143(4):1265–1267. [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Kilgus J., Doran D. M., Matile H., Etlinger H., Trzeciak A., Gillessen D., Pink J. R. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988 Dec 22;336(6201):778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- Young D., Kent L., Rees A., Lamb J., Ivanyi J. Immunological activity of a 38-kilodalton protein purified from Mycobacterium tuberculosis. Infect Immun. 1986 Oct;54(1):177–183. doi: 10.1128/iai.54.1.177-183.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


