Abstract
We wanted to re-examine the hypotheses that natural killer (NK) cells preferentially react with immature cells, and that they are not directed against major histocompatibility complex (MHC) gene products. Rat marrow cells could be separated according to maturity on a four-step discontinuous density gradient of Percoll. Almost all the immature bone marrow cells with progenitor activity, as measured in vivo in a diffusion chamber assay or in vitro in a granulocyte/macrophage colony-forming assay, resided within the lighter density cell fraction (density approximately 1.065). The higher density cells (density approximately 1.082) contained mainly the more mature, non-proliferative cells within the granulocyte series. NK and lymphokine-activated killer (LAK) cells from athymic rats, being devoid of T cells, efficiently killed low- as well as high-density bone marrow cells from a fully allogeneic and a MHC congenic rat strain, while little or no killing was observed against syngeneic bone marrow cell fractions. LAK cells also effectively inhibited granulocyte/macrophage colony formation from allogeneic bone marrow precursors in vitro, while stimulating colony formation from syngeneic bone marrow cells. The NK-mediated killing of allogeneic bone marrow cells was effectively inhibited by NK-sensitive tumour cells, while there was much less inhibition of the killing of tumour cells by allogeneic bone marrow cells. We conclude that NK cells recognize MHC incompatibilities on both immature and mature allogeneic bone marrow cells through recognition systems not related to T-cell receptors, and that allospecific killing can explain the contrasting effect of NK cells on allogeneic and syngeneic haematopoiesis.
Full text
PDF
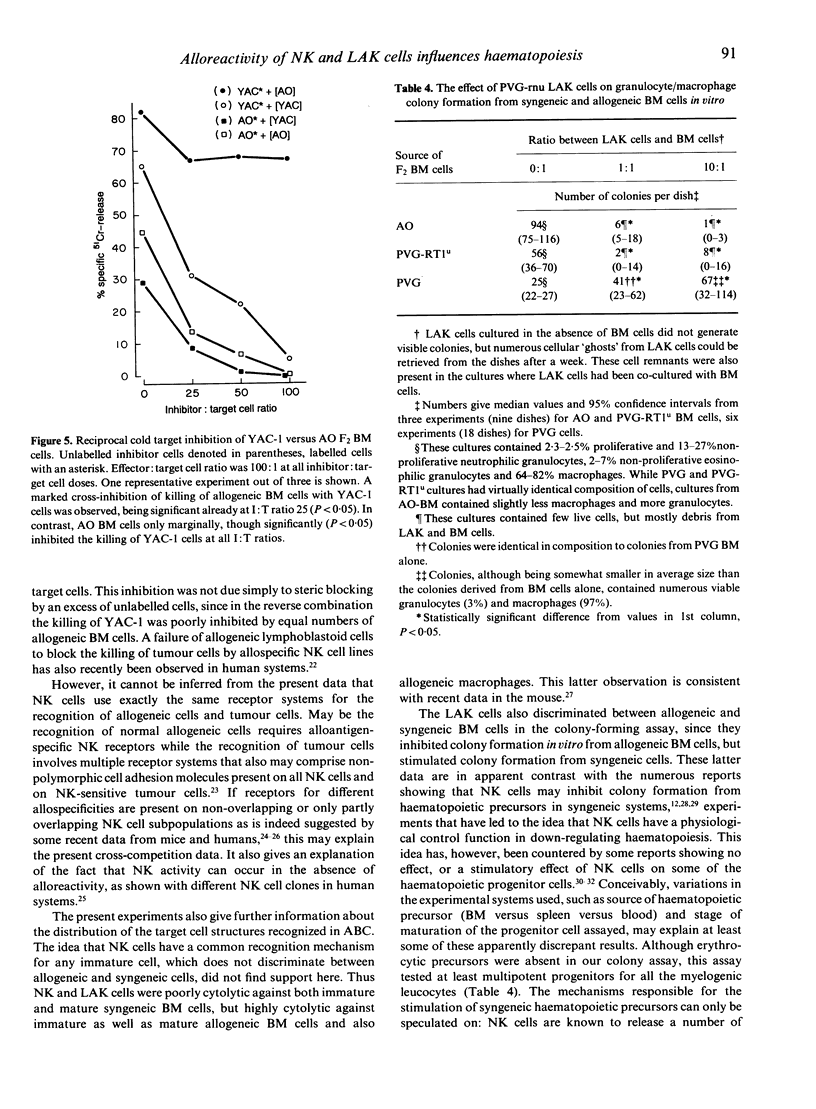


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Fajumi J., Sparshott S. M., Ford W. L., Butcher G. W. Major histocompatibility complex control of NK-related allogeneic lymphocyte cytotoxicity in rats. The contributions of strong and medial transplantation antigens. Transplantation. 1988 Nov;46(5):762–767. doi: 10.1097/00007890-198811000-00025. [DOI] [PubMed] [Google Scholar]
- Barlozzari T., Herberman R. B., Reynolds C. W. Inhibition of pluripotent hematopoietic stem cells of bone marrow by large granular lymphocytes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7691–7695. doi: 10.1073/pnas.84.21.7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benestad H. B. Formation of granulocytes and macrophages in diffusion chamber cultures of mouse blood leucocytes. Scand J Haematol. 1970;7(4):279–288. doi: 10.1111/j.1600-0609.1970.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Benestad H. B., Hersleth I. B., Rolstad B. Thymus and T cells are not essential for rat leucopoiesis. Eur J Haematol. 1989 Aug;43(2):150–157. doi: 10.1111/j.1600-0609.1989.tb00271.x. [DOI] [PubMed] [Google Scholar]
- Benestad H. B., Hersleth I. B., Strøm-Gundersen I. Methodological studies on growth of mouse granulocyte/macrophage colonies in methylcellulose-containing media. Int J Cell Cloning. 1984 Mar;2(2):113–125. doi: 10.1002/stem.5530020205. [DOI] [PubMed] [Google Scholar]
- Benestad H. B., Reikvam A. Diffusion chamber culturing of haematopoietic cells: methodological investigations and improvement of the technique. Exp Hematol. 1975 Aug;3(4):249–260. [PubMed] [Google Scholar]
- Benestad H. B., Strøm-Gundersen I. Flow cytometry of mouse bone marrow cells cultured in vivo or in vitro. Exp Hematol. 1982 Apr;10(4):343–351. [PubMed] [Google Scholar]
- Ciccone E., Pende D., Viale O., Tambussi G., Ferrini S., Biassoni R., Longo A., Guardiola J., Moretta A., Moretta L. Specific recognition of human CD3-CD16+ natural killer cells requires the expression of an autosomic recessive gene on target cells. J Exp Med. 1990 Jul 1;172(1):47–52. doi: 10.1084/jem.172.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone E., Viale O., Pende D., Malnati M., Biassoni R., Melioli G., Moretta A., Long E. O., Moretta L. Specific lysis of allogeneic cells after activation of CD3- lymphocytes in mixed lymphocyte culture. J Exp Med. 1988 Dec 1;168(6):2403–2408. doi: 10.1084/jem.168.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz G., Bennett M. Peculiar immunobiology of bone marrow allografts. II. Rejection of parental grafts by resistant F 1 hybrid mice. J Exp Med. 1971 Dec 1;134(6):1513–1528. doi: 10.1084/jem.134.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degliantoni G., Perussia B., Mangoni L., Trinchieri G. Inhibition of bone marrow colony formation by human natural killer cells and by natural killer cell-derived colony-inhibiting activity. J Exp Med. 1985 May 1;161(5):1152–1168. doi: 10.1084/jem.161.5.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissen E., Vaage J. T., Taskén K., Jahnsen T., Rolstad B., Fossum S. Alloreactive lymphokine-activated killer cells from athymic nude rats do not express CD3-associated alpha/beta or gamma/delta T cell receptors. Int Immunol. 1990;2(5):453–460. doi: 10.1093/intimm/2.5.453. [DOI] [PubMed] [Google Scholar]
- Ford W. L., Burr W., Simonsen M. A lymph node weight assay for the graft-versus-host activity of rat lymphoid cells. Transplantation. 1970 Sep;10(3):258–266. doi: 10.1097/00007890-197009000-00007. [DOI] [PubMed] [Google Scholar]
- Hansson M., Beran M., Andersson B., Kiessling R. Inhibition of in vitro granulopoiesis by autologous allogeneic human NK cells. J Immunol. 1982 Jul;129(1):126–132. [PubMed] [Google Scholar]
- Kiessling R., Hochman P. S., Haller O., Shearer G. M., Wigzell H., Cudkowicz G. Evidence for a similar or common mechanism for natural killer cell activity and resistance to hemopoietic grafts. Eur J Immunol. 1977 Sep;7(9):655–663. doi: 10.1002/eji.1830070915. [DOI] [PubMed] [Google Scholar]
- Lotzová E. Resistance to parental, allogeneic and xenogeneic hemopoietic grafts in irradiated mice. Exp Hematol. 1977 May;5(3):215–235. [PubMed] [Google Scholar]
- Lotzová E., Savary C. A., Pollack S. B. Prevention of rejection of allogeneic bone marrow transplants by NK 1.1 antiserum. Transplantation. 1983 May;35(5):490–494. doi: 10.1097/00007890-198305000-00019. [DOI] [PubMed] [Google Scholar]
- Matera L., Santoli D., Garbarino G., Pegoraro L., Bellone G., Pagliardi G. Modulation of in vitro myelopoiesis by LGL: different effects on early and late progenitor cells. J Immunol. 1986 Feb 15;136(4):1260–1265. [PubMed] [Google Scholar]
- Nagler A., Greenberg P. L., Lanier L. L., Phillips J. H. The effects of recombinant interleukin 2-activated natural killer cells on autologous peripheral blood hematopoietic progenitors. J Exp Med. 1988 Jul 1;168(1):47–54. doi: 10.1084/jem.168.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer C. M., Sieff C. A., Smith B. R., Ault K. A., Nathan D. G. Hematopoiesis in vitro coexists with natural killer lymphocytes. Blood. 1989 Nov 15;74(7):2376–2382. [PubMed] [Google Scholar]
- Pistoia V., Zupo S., Corcione A., Roncella S., Matera L., Ghio R., Ferrarini M. Production of colony-stimulating activity by human natural killer cells: analysis of the conditions that influence the release and detection of colony-stimulating activity. Blood. 1989 Jul;74(1):156–164. [PubMed] [Google Scholar]
- Reynolds C. W., Timonen T., Herberman R. B. Natural killer (NK) cell activity in the rat. I. Isolation and characterization of the effector cells. J Immunol. 1981 Jul;127(1):282–287. [PubMed] [Google Scholar]
- Rolstad B., Benestad H. B. The "natural resistance" to bone marrow allografts in normal and athymic nude rats. Rapid cytotoxic reactions both in vivo and in vitro. Eur J Immunol. 1984 Sep;14(9):793–799. doi: 10.1002/eji.1830140906. [DOI] [PubMed] [Google Scholar]
- Rolstad B., Fossum S. Allogeneic lymphocyte cytotoxicity (ALC) in rats: establishment of an in vitro assay, and direct evidence that cells with natural killer (NK) activity are involved in ALC. Immunology. 1987 Feb;60(2):151–157. [PMC free article] [PubMed] [Google Scholar]
- Rolstad B., Fossum S., Benestad H. B. The target cells for rat NK cells among allogeneic bone marrow cells. Adv Exp Med Biol. 1988;237:439–445. doi: 10.1007/978-1-4684-5535-9_67. [DOI] [PubMed] [Google Scholar]
- Rolstad B., Herberman R. B., Reynolds C. W. Natural killer cell activity in the rat. V. The circulation patterns and tissue localization of peripheral blood large granular lymphocytes (LGL). J Immunol. 1986 Apr 15;136(8):2800–2808. [PubMed] [Google Scholar]
- Sentman C. L., Hackett J., Jr, Kumar V., Bennett M. Identification of a subset of murine natural killer cells that mediates rejection of Hh-1d but not Hh-1b bone marrow grafts. J Exp Med. 1989 Jul 1;170(1):191–202. doi: 10.1084/jem.170.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura K., Kaminsky S. G., Nakamura I. Acute rejection of allogeneic hemopoietic progenitors by genetically resistant mice. Eur J Immunol. 1989 Jul;19(7):1165–1170. doi: 10.1002/eji.1830190702. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Bianchi E., Bass H., Suzuki T., Bender J., Pardi R., Brenner C. A., Larrick J. W., Engleman E. G. Natural killer lines and clones with apparent antigen specificity. J Exp Med. 1990 Aug 1;172(2):457–462. doi: 10.1084/jem.172.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimperis J. Z., Niemeyer C. M., Sieff C. A., Mathey-Prevot B., Nathan D. G., Arceci R. J. Granulocyte-macrophage colony-stimulating factor and interleukin-3 mRNAs are produced by a small fraction of blood mononuclear cells. Blood. 1989 Oct;74(5):1525–1530. [PubMed] [Google Scholar]



