Abstract
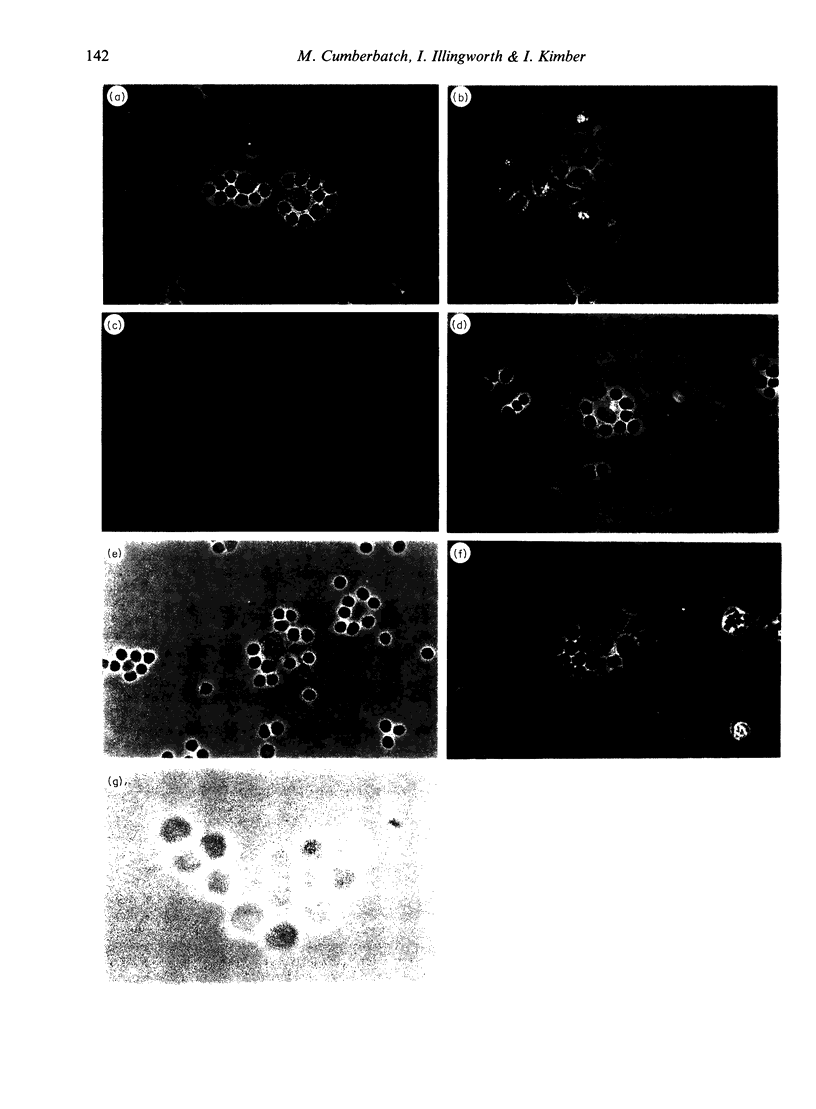
Following topical exposure to skin-sensitizing chemicals, Langerhans' cells, a significant proportion of which bear antigen, are induced to migrate from the epidermis to the regional lymph node. There is evidence that the antigen-bearing cells which arrive in the draining lymph nodes have the functional characteristics of mature dendritic cells (DC) and efficiently induce T-lymphocyte activation in vitro and contact sensitization in vivo. In contrast, freshly isolated Langerhans' cells are known to be relatively inefficient antigen-presenting cells. Evidence exists that during culture in the presence of granulocyte/macrophage colony-stimulating factor, Langerhans' cells undergo a functional maturation and assume the characteristics of dendritic cells. We have speculated that, in response to chemical exposure and the stimulus to migrate. Langerhans' cells undergo a similar maturation in vivo. To investigate this we have examined the capacity of draining lymph node DC to form antigen-independent clusters with T lymphocytes. Previous studies have confirmed that freshly isolated Langerhans' cells are unable to form such clusters. We report, however, that the antigen-bearing DC which arrive in the draining lymph nodes following skin sensitization, and which are recently derived from epidermal Langerhans' cells, efficiently form clusters with lymphocytes. Thus, antigen-bearing DC were found to have formed clusters with lymphocytes in situ in the draining lymph node, and to readily form clusters with lymphocytes in vitro. In both cases a higher proportion of lymphocytes associated with DC in clusters were T cells. An interesting observation was that DC within draining nodes appeared more efficient at cluster formation than DC in resting nodes, and that within draining nodes antigen-bearing DC formed clusters with greater affinity and/or greater stability than DC which lacked antigen. Taken together these data demonstrate that Langerhans' cell-derived antigen-bearing cells which accumulate in the draining lymph nodes following skin sensitization form clusters with lymphocytes in the manner of mature DC. This is compatible with the hypothesis that, while in transit from the skin, Langerhans' cells are subject to a functional maturation comparable to that witnessed in vitro.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Weinstein D. E., Steinman R. M. Clustering with dendritic cells precedes and is essential for T-cell proliferation in a mitogenesis model. Immunology. 1988 Apr;63(4):691–696. [PMC free article] [PubMed] [Google Scholar]
- Breel M., Mebius R., Kraal G. The interaction of interdigitating cells and lymphocytes in vitro and in vivo. Immunology. 1988 Feb;63(2):331–337. [PMC free article] [PubMed] [Google Scholar]
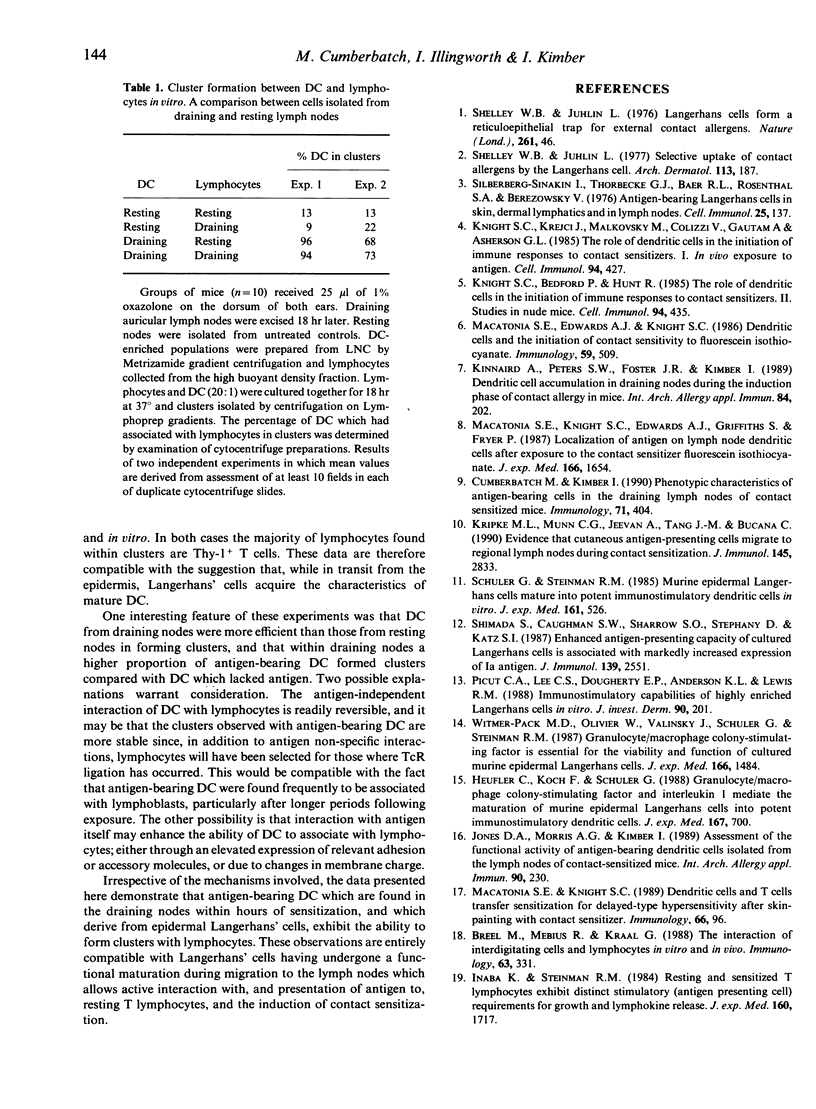
- Cumberbatch M., Kimber I. Phenotypic characteristics of antigen-bearing cells in the draining lymph nodes of contact sensitized mice. Immunology. 1990 Nov;71(3):404–410. [PMC free article] [PubMed] [Google Scholar]
- Flechner E. R., Freudenthal P. S., Kaplan G., Steinman R. M. Antigen-specific T lymphocytes efficiently cluster with dendritic cells in the human primary mixed-leukocyte reaction. Cell Immunol. 1988 Jan;111(1):183–195. doi: 10.1016/0008-8749(88)90062-7. [DOI] [PubMed] [Google Scholar]
- Green J., Jotte R. Interactions between T helper cells and dendritic cells during the rat mixed lymphocyte reaction. J Exp Med. 1985 Nov 1;162(5):1546–1560. doi: 10.1084/jem.162.5.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heufler C., Koch F., Schuler G. Granulocyte/macrophage colony-stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J Exp Med. 1988 Feb 1;167(2):700–705. doi: 10.1084/jem.167.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Granelli-Piperno A., Steinman R. M. Dendritic cells induce T lymphocytes to release B cell-stimulating factors by an interleukin 2-dependent mechanism. J Exp Med. 1983 Dec 1;158(6):2040–2057. doi: 10.1084/jem.158.6.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Schuler G., Witmer M. D., Valinksy J., Atassi B., Steinman R. M. Immunologic properties of purified epidermal Langerhans cells. Distinct requirements for stimulation of unprimed and sensitized T lymphocytes. J Exp Med. 1986 Aug 1;164(2):605–613. doi: 10.1084/jem.164.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Accessory cell-T lymphocyte interactions. Antigen-dependent and -independent clustering. J Exp Med. 1986 Feb 1;163(2):247–261. doi: 10.1084/jem.163.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Monoclonal antibodies to LFA-1 and to CD4 inhibit the mixed leukocyte reaction after the antigen-dependent clustering of dendritic cells and T lymphocytes. J Exp Med. 1987 May 1;165(5):1403–1417. doi: 10.1084/jem.165.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Resting and sensitized T lymphocytes exhibit distinct stimulatory (antigen-presenting cell) requirements for growth and lymphokine release. J Exp Med. 1984 Dec 1;160(6):1717–1735. doi: 10.1084/jem.160.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. A., Morris A. G., Kimber I. Assessment of the functional activity of antigen-bearing dendritic cells isolated from the lymph nodes of contact-sensitized mice. Int Arch Allergy Appl Immunol. 1989;90(3):230–236. doi: 10.1159/000235030. [DOI] [PubMed] [Google Scholar]
- Kinnaird A., Peters S. W., Foster J. R., Kimber I. Dendritic cell accumulation in draining lymph nodes during the induction phase of contact allergy in mice. Int Arch Allergy Appl Immunol. 1989;89(2-3):202–210. doi: 10.1159/000234947. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Bedford P., Hunt R. The role of dendritic cells in the initiation of immune responses to contact sensitizers. II. Studies in nude mice. Cell Immunol. 1985 Sep;94(2):435–439. doi: 10.1016/0008-8749(85)90267-9. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Krejci J., Malkovsky M., Colizzi V., Gautam A., Asherson G. L. The role of dendritic cells in the initiation of immune responses to contact sensitizers. I. In vivo exposure to antigen. Cell Immunol. 1985 Sep;94(2):427–434. doi: 10.1016/0008-8749(85)90266-7. [DOI] [PubMed] [Google Scholar]
- Koide S. L., Inaba K., Steinman R. M. Interleukin 1 enhances T-dependent immune responses by amplifying the function of dendritic cells. J Exp Med. 1987 Feb 1;165(2):515–530. doi: 10.1084/jem.165.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke M. L., Munn C. G., Jeevan A., Tang J. M., Bucana C. Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization. J Immunol. 1990 Nov 1;145(9):2833–2838. [PubMed] [Google Scholar]
- Kupper T. S., Lee F., Coleman D., Chodakewitz J., Flood P., Horowitz M. Keratinocyte derived T-cell growth factor (KTGF) is identical to granulocyte macrophage colony stimulating factor (GM-CSF). J Invest Dermatol. 1988 Aug;91(2):185–188. doi: 10.1111/1523-1747.ep12464470. [DOI] [PubMed] [Google Scholar]
- Macatonia S. E., Edwards A. J., Knight S. C. Dendritic cells and the initiation of contact sensitivity to fluorescein isothiocyanate. Immunology. 1986 Dec;59(4):509–514. [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Knight S. C. Dendritic cells and T cells transfer sensitization for delayed-type hypersensitivity after skin painting with contact sensitizer. Immunology. 1989 Jan;66(1):96–99. [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Knight S. C., Edwards A. J., Griffiths S., Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987 Dec 1;166(6):1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picut C. A., Lee C. S., Dougherty E. P., Anderson K. L., Lewis R. M. Immunostimulatory capabilities of highly enriched Langerhans cells in vitro. J Invest Dermatol. 1988 Feb;90(2):201–206. doi: 10.1111/1523-1747.ep12462221. [DOI] [PubMed] [Google Scholar]
- Schuler G., Steinman R. M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985 Mar 1;161(3):526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley W. B., Juhlin L. Langerhans cells form a reticuloepithelial trap for external contact antigens. Nature. 1976 May 6;261(5555):46–47. doi: 10.1038/261046a0. [DOI] [PubMed] [Google Scholar]
- Shelley W. B., Juhlin L. Selective uptake of contact allergens by the Langerhans cell. Arch Dermatol. 1977 Feb;113(2):187–192. [PubMed] [Google Scholar]
- Shimada S., Caughman S. W., Sharrow S. O., Stephany D., Katz S. I. Enhanced antigen-presenting capacity of cultured Langerhans' cells is associated with markedly increased expression of Ia antigen. J Immunol. 1987 Oct 15;139(8):2551–2555. [PubMed] [Google Scholar]
- Silberberg-Sinakin I., Thorbecke G. J., Baer R. L., Rosenthal S. A., Berezowsky V. Antigen-bearing langerhans cells in skin, dermal lymphatics and in lymph nodes. Cell Immunol. 1976 Aug;25(2):137–151. doi: 10.1016/0008-8749(76)90105-2. [DOI] [PubMed] [Google Scholar]
- Witmer-Pack M. D., Olivier W., Valinsky J., Schuler G., Steinman R. M. Granulocyte/macrophage colony-stimulating factor is essential for the viability and function of cultured murine epidermal Langerhans cells. J Exp Med. 1987 Nov 1;166(5):1484–1498. doi: 10.1084/jem.166.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]



