Abstract
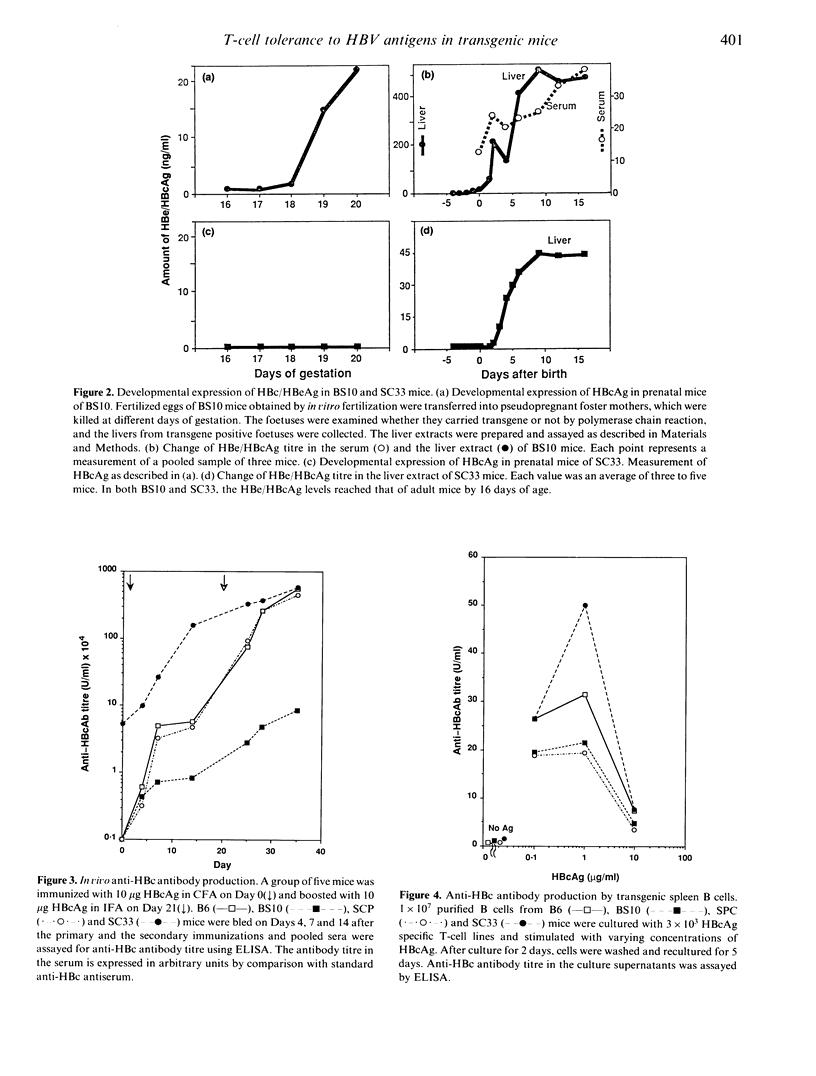
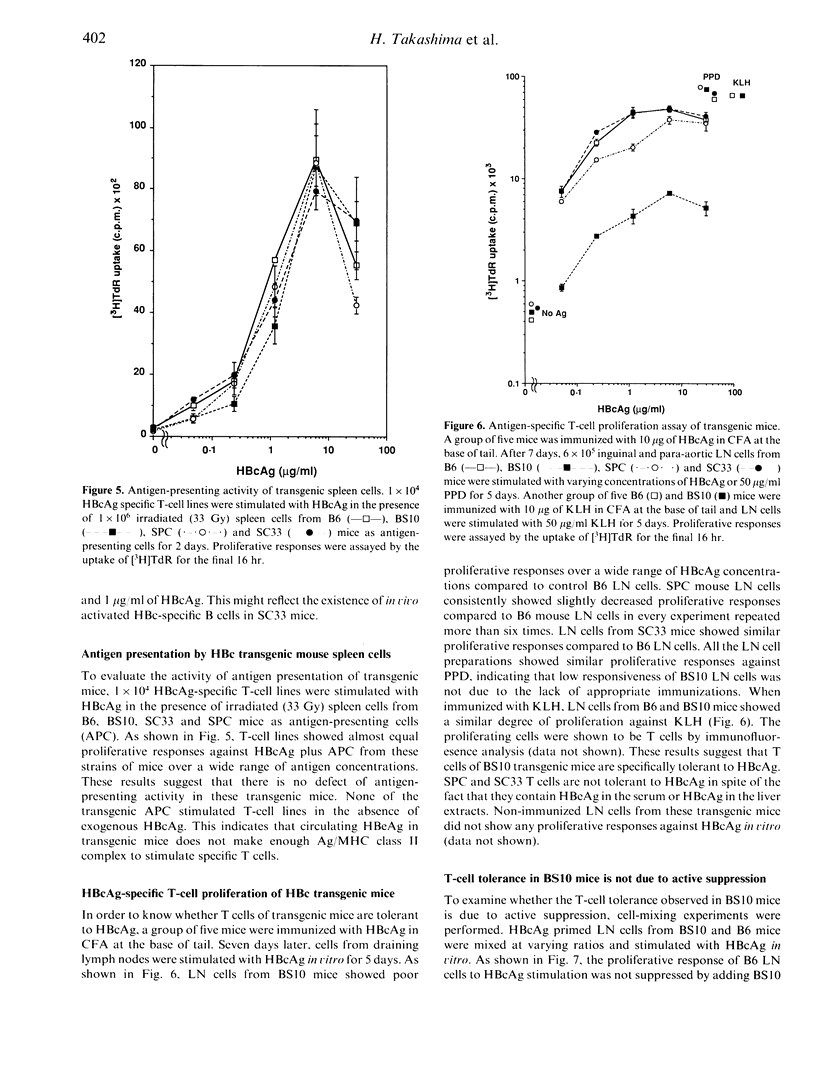
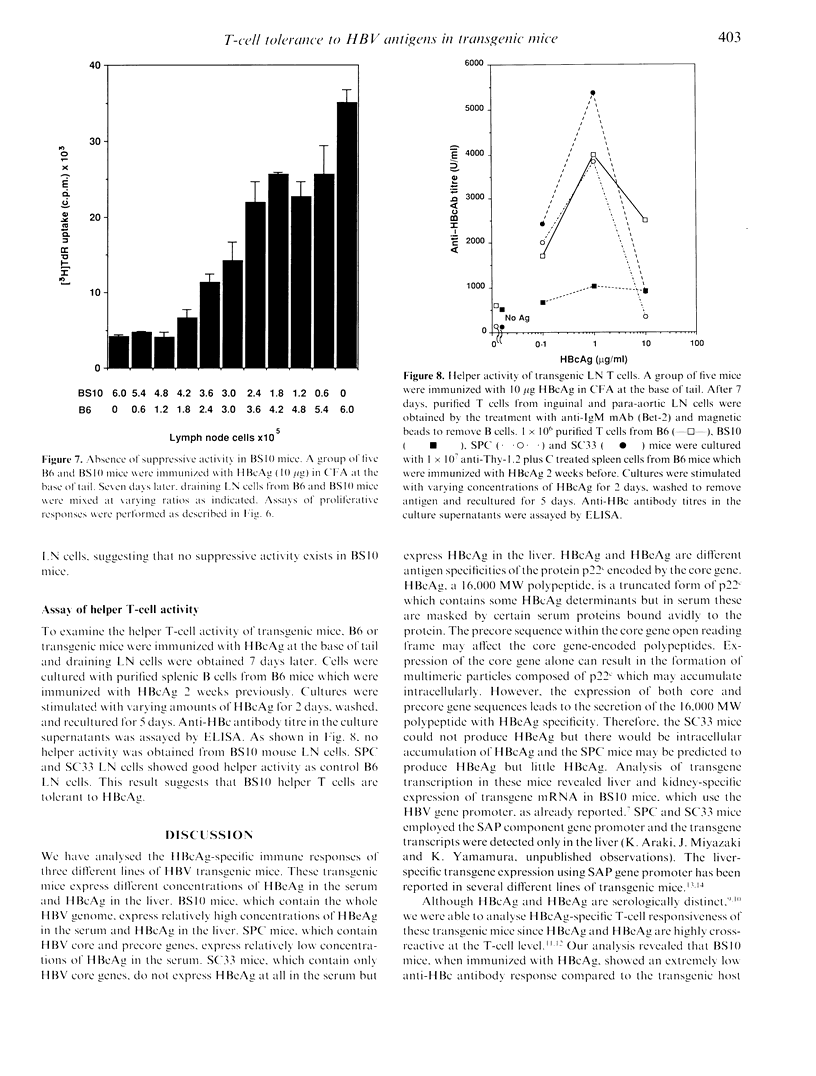
We made three different lines of hepatitis B virus (HBV) transgenic mice which express different amounts of hepatitis B e antigen (HBeAg) and/or hepatitis B core antigen (HBcAg) to analyse the cellular mechanisms of HBcAg specific T-cell tolerance. BS10 (official designation, 1.2HB-BS10) transgenic mice, which contain the whole HBV genome, express relatively high amounts of HBeAg in the serum and HBcAg in the liver. SPC mice, which contain hepatitis B virus core and precore gene, express small amounts of HBeAg in the serum but not HBcAg in the liver. SC33 mice, which contain only hepatitis B core gene, do not express HBeAg in the serum but express HBcAg in the liver. BS10 mice showed a very low anti-HBc antibody response after primary and secondary immunizations with recombinant HBcAg compared to transgenic host C57BL/6 (B6) mice. SPC mice showed an almost equal level of anti-HBc antibody response compared to B6 mice. SC33 mice contained anti-HBc antibody even before immunization and showed high titres of anti-HBc antibody response after immunization with HBcAg. Analysis of cellular site(s) of low responsiveness of BS10 mice revealed that proliferating and helper T cells are specifically tolerant to HBcAg. B cells and antigen-presenting cells in BS10 mice were not defective. SC33, SPC and BS10 mice differ a little in their developmental expression of HBc/HBeAg. Our results suggest critical roles of the nature (circulating versus non-circulating) as well as the time of expression of self-antigens in T-cell tolerance.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki K., Hino O., Miyazaki J., Yamamura K. Development of two types of hepatocellular carcinoma in transgenic mice carrying the SV40 large T-antigen gene. Carcinogenesis. 1991 Nov;12(11):2059–2062. doi: 10.1093/carcin/12.11.2059. [DOI] [PubMed] [Google Scholar]
- Araki K., Miyazaki J., Hino O., Tomita N., Chisaka O., Matsubara K., Yamamura K. Expression and replication of hepatitis B virus genome in transgenic mice. Proc Natl Acad Sci U S A. 1989 Jan;86(1):207–211. doi: 10.1073/pnas.86.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K., Nishimura S., Ochiya T., Okubo K., Miyazaki J., Matsubara K., Yamamura K. Production and effect of infectious Dane particles in transgenic mice. Jpn J Cancer Res. 1991 Mar;82(3):235–239. doi: 10.1111/j.1349-7006.1991.tb01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkly L. C., Lo D., Kanagawa O., Brinster R. L., Flavell R. A. T-cell tolerance by clonal anergy in transgenic mice with nonlymphoid expression of MHC class II I-E. Nature. 1989 Nov 30;342(6249):564–566. doi: 10.1038/342564a0. [DOI] [PubMed] [Google Scholar]
- Chisari F. V., Filippi P., Buras J., McLachlan A., Popper H., Pinkert C. A., Palmiter R. D., Brinster R. L. Structural and pathological effects of synthesis of hepatitis B virus large envelope polypeptide in transgenic mice. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6909–6913. doi: 10.1073/pnas.84.19.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari F. V., Klopchin K., Moriyama T., Pasquinelli C., Dunsford H. A., Sell S., Pinkert C. A., Brinster R. L., Palmiter R. D. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell. 1989 Dec 22;59(6):1145–1156. doi: 10.1016/0092-8674(89)90770-8. [DOI] [PubMed] [Google Scholar]
- Fujiyama A., Miyanohara A., Nozaki C., Yoneyama T., Ohtomo N., Matsubara K. Cloning and structural analyses of hepatitis B virus DNAs, subtype adr. Nucleic Acids Res. 1983 Jul 11;11(13):4601–4610. doi: 10.1093/nar/11.13.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan S., Aiso S., Michie S. A., McDevitt H. O. Immune deficiency due to high copy numbers of an Ak beta transgene. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7319–7323. doi: 10.1073/pnas.87.18.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga T., Wakasugi S., Inomoto T., Uehira M., Ohnishi S., Nishiguchi S., Araki K., Uno M., Miyazaki J., Maeda S. Liver-specific and high-level expression of human serum amyloid P component gene in transgenic mice. Dev Genet. 1989;10(5):365–371. doi: 10.1002/dvg.1020100504. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Milich D. R., Jones J. E., Hughes J. L., Price J., Raney A. K., McLachlan A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci U S A. 1990 Sep;87(17):6599–6603. doi: 10.1073/pnas.87.17.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Moriarty A., Thornton G. B. Immune response to hepatitis B virus core antigen (HBcAg): localization of T cell recognition sites within HBcAg/HBeAg. J Immunol. 1987 Aug 15;139(4):1223–1231. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Stahl S., Wingfield P., Thornton G. B., Hughes J. L., Jones J. E. Comparative immunogenicity of hepatitis B virus core and E antigens. J Immunol. 1988 Nov 15;141(10):3617–3624. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science. 1986 Dec 12;234(4782):1398–1401. doi: 10.1126/science.3491425. [DOI] [PubMed] [Google Scholar]
- Mineta T., Matsunaga M., Takashima H., Uno M., Miyazaki J., Yamamura K., Kimoto M. (BALB/c x C57BL/6)F1 mice are tolerant to undetectable mixed haplotype A beta dA alpha b and mixed isotype A beta dE alpha d self class II molecules. Int Immunol. 1991 May;3(5):453–460. doi: 10.1093/intimm/3.5.453. [DOI] [PubMed] [Google Scholar]
- Moriyama T., Guilhot S., Klopchin K., Moss B., Pinkert C. A., Palmiter R. D., Brinster R. L., Kanagawa O., Chisari F. V. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science. 1990 Apr 20;248(4953):361–364. doi: 10.1126/science.1691527. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. Cellular mechanisms of immunologic tolerance. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- Ohnishi S., Maeda S., Shimada K., Arao T. Isolation and characterization of the complete complementary and genomic DNA sequences of human serum amyloid P component. J Biochem. 1986 Oct;100(4):849–858. doi: 10.1093/oxfordjournals.jbchem.a121797. [DOI] [PubMed] [Google Scholar]
- Pircher H., Rohrer U. H., Moskophidis D., Zinkernagel R. M., Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991 Jun 6;351(6326):482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Machida A., Funatsu G., Nomura M., Usuda S., Aoyagi S., Tachibana K., Miyamoto H., Imai M., Nakamura T. Immunochemical structure of hepatitis B e antigen in the serum. J Immunol. 1983 Jun;130(6):2903–2907. [PubMed] [Google Scholar]
- Whiteley P. J., Poindexter N. J., Landon C., Kapp J. A. A peripheral mechanism preserves self-tolerance to a secreted protein in transgenic mice. J Immunol. 1990 Sep 1;145(5):1376–1381. [PubMed] [Google Scholar]


