Abstract
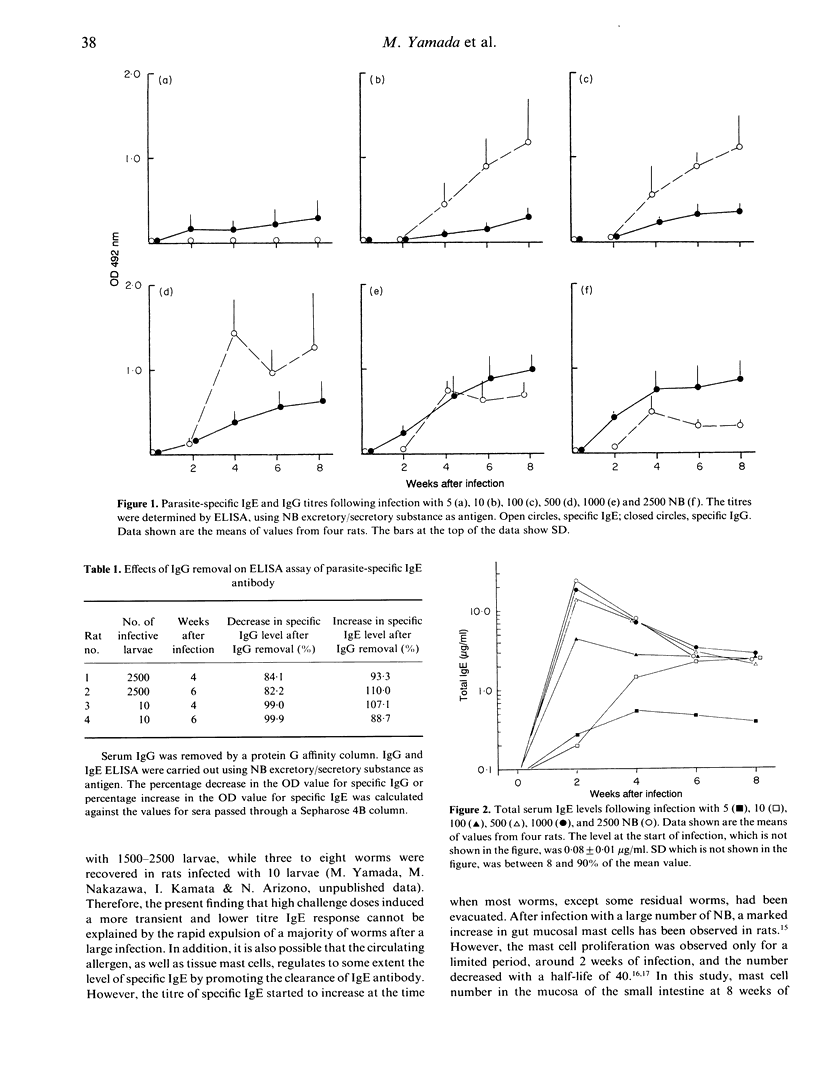
Specific and non-specific IgE antibody responses were studied in SD rats infected with between 5 and 2500 Nippostrongylus brasiliensis (NB) larvae. In rats with 2500 NB larvae, specific IgE antibody, measured by enzyme-linked immunosorbent assay (ELISA) using NB excretory/secretory substance as antigen, reached a peak at 4 weeks of infection and gradually declined. On the other hand, in rats infected with 10 or 100 NB larvae, specific IgE was induced at 4 weeks of infection and the level continued to rise until at least 8 weeks after infection. The level at 8 weeks was significantly higher in rats infected with 10 or 100 larvae than in rats infected with 2500 larvae. The results indicate that the low-level infection induced a much more sustained specific IgE response than that induced after heavy infection. However, the level of specific IgG was correlated with the dose of infection, and reached a plateau 6 weeks after infection. Total serum IgE increased significantly even in rats infected with five larvae, a dose which did not induce detectable specific IgE. The kinetics of the production of total IgE was different in rats with light and heavy infections. In rats infected with five or 10 larvae, total IgE increased slowly and reached a plateau 4 weeks after infection. On the other hand, rats infected with more than 500 larvae showed a remarkable rise in total IgE at 2 weeks of infection; this rise gradually declined thereafter. Six weeks after infection, total IgE levels were almost the same (2-3 micrograms/ml) in rats infected with 10-2500 NB larvae. These results show that low-level NB infection induces a significant and sustained specific and non-specific IgE response in rats.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalberse R. C., van der Gaag R., van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983 Feb;130(2):722–726. [PubMed] [Google Scholar]
- Arizono N., Nakao S. Kinetics and staining properties of mast cells proliferating in rat small intestine tunica muscularis and subserosa following infection with Nippostrongylus brasiliensis. APMIS. 1988 Nov;96(11):964–970. doi: 10.1111/j.1699-0463.1988.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Arizono N., Shiota T., Yamada M., Matsumoto Y., Yoshikawa H., Matsuda S., Tegoshi T. Bromodeoxyuridine labeling studies on the proliferation of intestinal mucosal mast cells in normal and athymic rats. APMIS. 1990 Apr;98(4):369–376. doi: 10.1111/j.1699-0463.1990.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Carson D., Metzger H., Bloch K. J. Serum IgE levels during the potentiated reagin response to egg albumin in rats infected with Nippostrongylus brasiliensis. J Immunol. 1975 Jan;114(1 Pt 2):521–523. [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Dessaint J. P., Bout D., Wattre P., Capron A. Quantitative determination of specific IgE antibodies to Echinococcus granulosus and IgE levels in sera from patients with hydatid disease. Immunology. 1975 Nov;29(5):813–823. [PMC free article] [PubMed] [Google Scholar]
- Diaz-Sanchez D., Kemeny D. M. Generation of a long-lived IgE response in high and low responder strains of rat by co-administration of ricin and antigen. Immunology. 1991 Feb;72(2):297–303. [PMC free article] [PubMed] [Google Scholar]
- Diaz-Sanchez D., Kemeny D. M. The sensitivity of rat CD8+ and CD4+ T cells to ricin in vivo and in vitro and their relationship to IgE regulation. Immunology. 1990 Jan;69(1):71–77. [PMC free article] [PubMed] [Google Scholar]
- Hagan P., Blumenthal U. J., Dunn D., Simpson A. J., Wilkins H. A. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991 Jan 17;349(6306):243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- Hamilton R. G., Hussain R., Ottesen E. A., Adkinson N. F., Jr The quantitation of parasite-specific human IgG and IgE in sera: evaluation of solid-phase RIA and ELISA methodology. J Immunol Methods. 1981;44(1):101–114. doi: 10.1016/0022-1759(81)90111-3. [DOI] [PubMed] [Google Scholar]
- Hussain R., Ottesen E. A. IgE responses in human filariasis. IV. Parallel antigen recognition by IgE and IgG4 subclass antibodies. J Immunol. 1986 Mar 1;136(5):1859–1863. [PubMed] [Google Scholar]
- Ishizaka T., Urban J. F., Jr, Ishizaka K. IgE formation in the rat following infection with Nippostrongylus brasiliensis. I. Proliferation and differentiation of IgE-bearing cells. Cell Immunol. 1976 Mar 15;22(2):248–261. doi: 10.1016/0008-8749(76)90027-7. [DOI] [PubMed] [Google Scholar]
- Jarrett E. E., Bazin H. Serum immunoglobulin levels in N. brasiliensis infection. Clin Exp Immunol. 1977 Nov;30(2):330–332. [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E., Haig D. M. Time course studies on rat IgE production in N. Brasiliensis infection. Clin Exp Immunol. 1976 May;24(2):346–351. [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E., Jarrett W. F., Urquhart G. M. Quantitative studies on the kinetics of establishment and expulsion of intestinal nematode populations in susceptible and immune hosts. Nippostrongylus brasiliensis in the rat. Parasitology. 1968 Aug;58(3):625–639. doi: 10.1017/s0031182000028924. [DOI] [PubMed] [Google Scholar]
- Jarrett E. E., Miller H. R. Production and activities of IgE in helminth infection. Prog Allergy. 1982;31:178–233. [PubMed] [Google Scholar]
- Jarrett E. E., Stewart D. C. Rat IgE production. I. Effect of dose of antigen on primary and secondary reaginic antibody responses. Immunology. 1974 Sep;27(3):365–381. [PMC free article] [PubMed] [Google Scholar]
- Jarrett E., Bazin H. Elevation of total serum IgE in rats following helminth parasite infection. Nature. 1974 Oct 18;251(5476):613–614. doi: 10.1038/251613a0. [DOI] [PubMed] [Google Scholar]
- Lebrun P., Spiegelberg H. L. Concomitant immunoglobulin E and immunoglobulin G1 formation in Nippostrongylus brasiliensis-infected mice. J Immunol. 1987 Sep 1;139(5):1459–1465. [PubMed] [Google Scholar]
- Lichtenstein L. M., Holtzman N. A., Burnett L. S. A quantitative in vitro study of the chromatographic distribution and immunoglobulin characteristics of human blocking antibody. J Immunol. 1968 Aug;101(2):317–324. [PubMed] [Google Scholar]
- Miller H. R., Jarrett W. F. Immune reactions in mucous membranes. I. Intestinal mast cell response during helminth expulsion in the rat. Immunology. 1971 Mar;20(3):277–288. [PMC free article] [PubMed] [Google Scholar]
- Ogilvie B. M., Jones V. E. Parasitological review. Nippostrongylus brasiliensis: a review of immunity and host-parasite relationship in the rat. Exp Parasitol. 1971 Feb;29(1):138–177. doi: 10.1016/0014-4894(71)90021-x. [DOI] [PubMed] [Google Scholar]
- Ogilvie B. M. Reagin-like antibodies in rats infected with the nematode parasite Nippostrongylus brasiliensis. Immunology. 1967 Feb;12(2):113–131. [PMC free article] [PubMed] [Google Scholar]
- Orr T. S., Blair A. M. Potentiated reagin response to egg albumin and conalbumin in Nippostrongylus brasiliensis infected rats. Life Sci. 1969 Oct 15;8(20):1073–1077. doi: 10.1016/0024-3205(69)90459-7. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Regulation and deregulation of human IgE synthesis. Immunol Today. 1990 Sep;11(9):316–321. doi: 10.1016/s0167-5699(10)80004-0. [DOI] [PubMed] [Google Scholar]
- Stanworth D. R., Smith A. K. Inhibition of reagin-mediated PCA reactions in baboons by the human IgG4 sub-class. Clin Allergy. 1973 Mar;3(1):37–41. doi: 10.1111/j.1365-2222.1973.tb01307.x. [DOI] [PubMed] [Google Scholar]
- Tada T., Okumura K., Taniguchi M. Regulation of homocytotropic antibody formation in the rat. VII. Carrier functions in the anti-hapten homocytotropic antibody response. J Immunol. 1972 Jun;108(6):1535–1541. [PubMed] [Google Scholar]
- Takatsu K., Ishizaka K. Reaginic antibody formation in the mouse. VII. Depression of the ongoing IgE antibody formation by suppressor T cells. J Immunol. 1976 Oct;117(4):1211–1218. [PubMed] [Google Scholar]
- Turner K. J., Feddema L., Quinn E. H. Non-specific potentiation of IgE by parasitic infections in man. Int Arch Allergy Appl Immunol. 1979;58(2):232–236. doi: 10.1159/000232197. [DOI] [PubMed] [Google Scholar]
- Vaz E. M., Vaz N. M., Levine B. B. Persistent formation of reagins in mice injected with low doses of ovalbuminl. Immunology. 1971 Jul;21(1):11–15. [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Nakazawa M., Matsumoto Y., Arizono N. IgE antibody production in rats against multiple components of excretory-secretory products of the nematode Nippostrongylus brasiliensis. Immunology. 1991 Jan;72(1):104–108. [PMC free article] [PubMed] [Google Scholar]


