Abstract
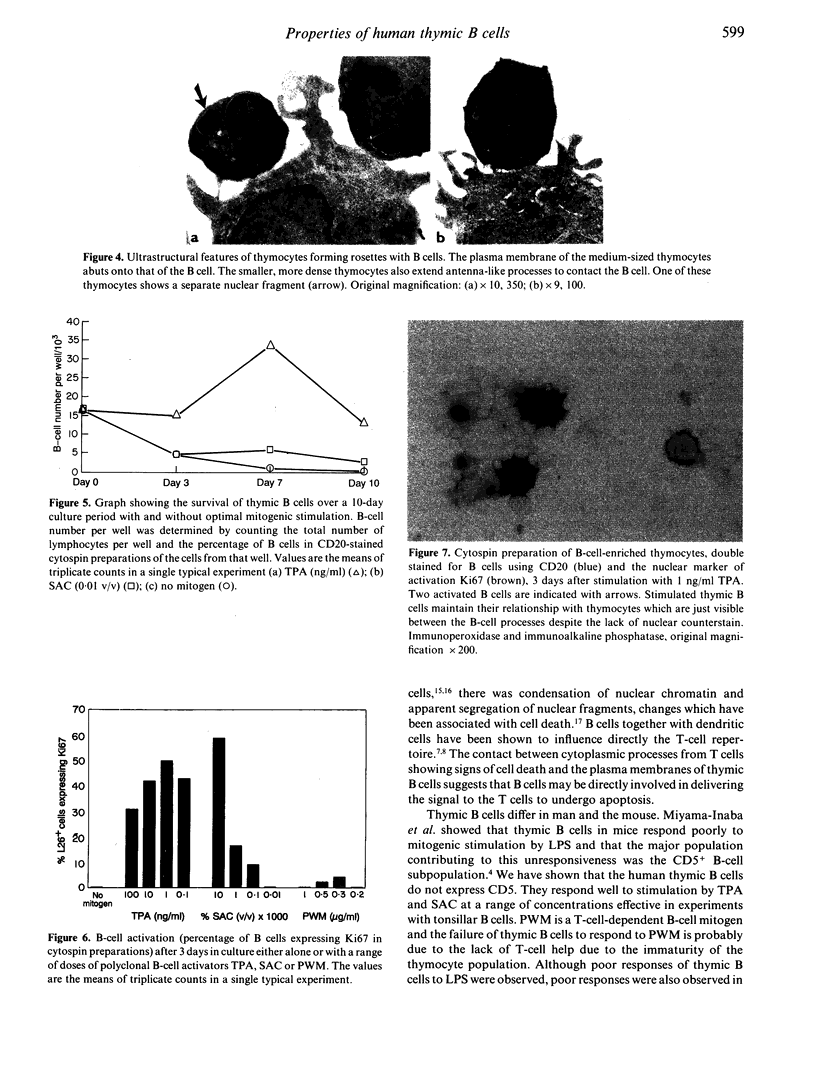
B cells, distinct from those seen in myasthenia gravis, are present in normal human thymic medulla, concentrated around the Hassall's corpuscles. We have shown that they constitute 33 +/- 4.8% of the total cells in the thymic medulla. In tissue sections they were often seen to have rosettes of thymocytes around them, a relationship which was maintained when the cells were isolated from the thymus. Thymic B cells expressed cytoplasmic immunoglobulins IgD, IgM and IgG but only rarely IgA. Unlike murine thymic B cells, human thymic B cells were CD5-. Freshly isolated thymic B cells were activated cells, but they rapidly became quiescent and died in culture over a 10-day period unless stimulated with mitogens. Thymic B cells responded to polyclonal B-cell activators SAC and TPA and when stimulated, maintained their relationship with thymocytes. Electron microscopic studies showed that two morphologically different thymocyte populations associated with the B cells. The plasma membranes of larger thymocytes were juxtaposed to the B-cell membrane, but smaller thymocytes with darker cytoplasm were associated with the B cells via cytoplasmic strands. Studies in mice have suggested that B cells are involved in thymic negative selection. The close association between activated B cells and thymocytes observed in this study supports this hypothesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu-Sánchez J. L., Faro J., Alonso J. M., Paige C. J., Martínez C., Marcos M. A. Ontogenic characterization of thymic B lymphocytes. Analysis in different mouse strains. Eur J Immunol. 1990 Aug;20(8):1767–1773. doi: 10.1002/eji.1830200822. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Fend F., Nachbaur D., Oberwasserlechner F., Kreczy A., Huber H., Müller-Hermelink H. K. Phenotype and topography of human thymic B cells. An immunohistologic study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60(6):381–388. doi: 10.1007/BF02899570. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Hofmann W. J., Momburg F., Möller P., Otto H. F. Intra- and extrathymic B cells in physiologic and pathologic conditions. Immunohistochemical study on normal thymus and lymphofollicular hyperplasia of the thymus. Virchows Arch A Pathol Anat Histopathol. 1988;412(5):431–442. doi: 10.1007/BF00750577. [DOI] [PubMed] [Google Scholar]
- Isaacson P. G., Norton A. J., Addis B. J. The human thymus contains a novel population of B lymphocytes. Lancet. 1987 Dec 26;2(8574):1488–1491. doi: 10.1016/s0140-6736(87)92622-5. [DOI] [PubMed] [Google Scholar]
- Ivanyi L., Lehner T. Stimulation of human lymphocytes by B-cell mitogens. Clin Exp Immunol. 1974 Nov;18(3):347–356. [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Mason D. Y., Comans-Bitter W. M., Cordell J. L., Verhoeven M. A., van Dongen J. J. Antibody L26 recognizes an intracellular epitope on the B-cell-associated CD20 antigen. Am J Pathol. 1990 Jun;136(6):1215–1222. [PMC free article] [PubMed] [Google Scholar]
- Mazda O., Watanabe Y., Gyotoku J., Katsura Y. Requirement of dendritic cells and B cells in the clonal deletion of Mls-reactive T cells in the thymus. J Exp Med. 1991 Mar 1;173(3):539–547. doi: 10.1084/jem.173.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyama-Inaba M., Kuma S., Inaba K., Ogata H., Iwai H., Yasumizu R., Muramatsu S., Steinman R. M., Ikehara S. Unusual phenotype of B cells in the thymus of normal mice. J Exp Med. 1988 Aug 1;168(2):811–816. doi: 10.1084/jem.168.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nango K., Inaba M., Inaba K., Adachi Y., Than S., Ishida T., Kumamoto T., Uyama M., Ikehara S. Ontogeny of thymic B cells in normal mice. Cell Immunol. 1991 Mar;133(1):109–115. doi: 10.1016/0008-8749(91)90183-c. [DOI] [PubMed] [Google Scholar]
- Scott R. J., Hall P. A., Haldane J. S., van Noorden S., Price Y., Lane D. P., Wright N. A. A comparison of immunohistochemical markers of cell proliferation with experimentally determined growth fraction. J Pathol. 1991 Oct;165(2):173–178. doi: 10.1002/path.1711650213. [DOI] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Mason D. Y. The normal and malignant germinal centre. Clin Haematol. 1982 Oct;11(3):531–559. [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- Zöller M. Intrathymic presentation of nominal antigen by B cells. Int Immunol. 1990;2(5):427–434. doi: 10.1093/intimm/2.5.427. [DOI] [PubMed] [Google Scholar]