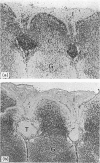
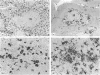
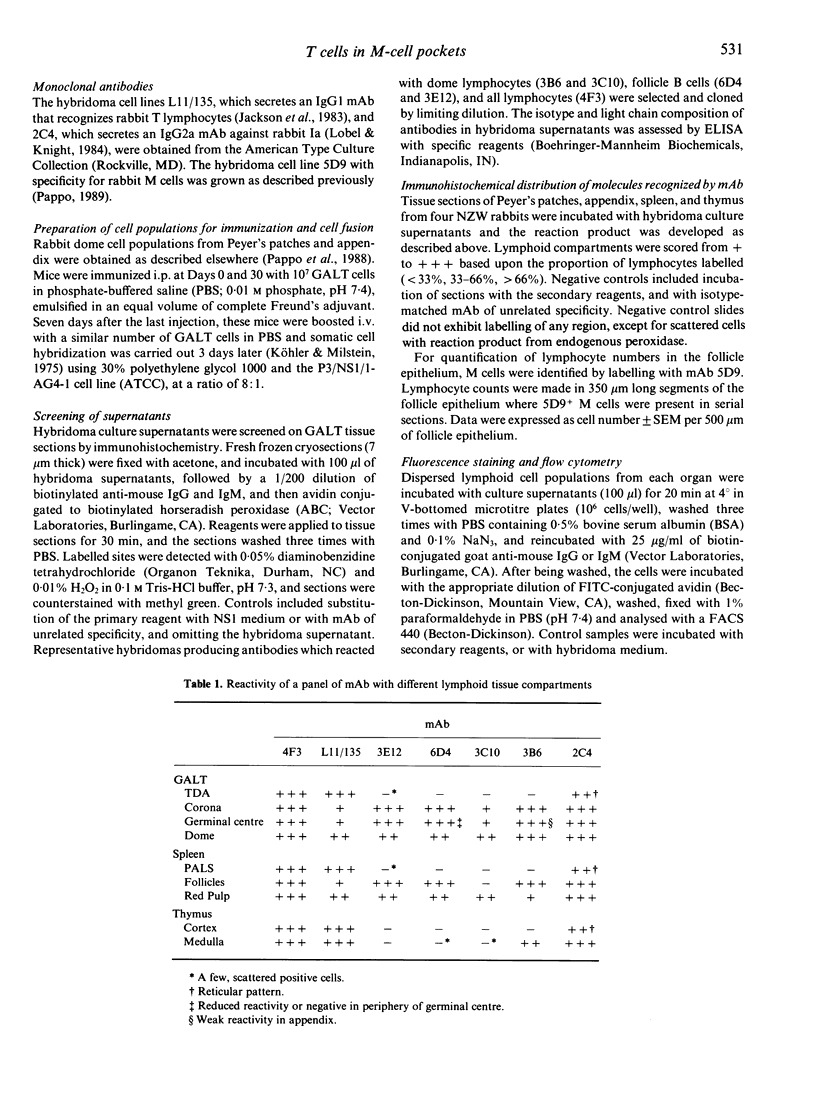
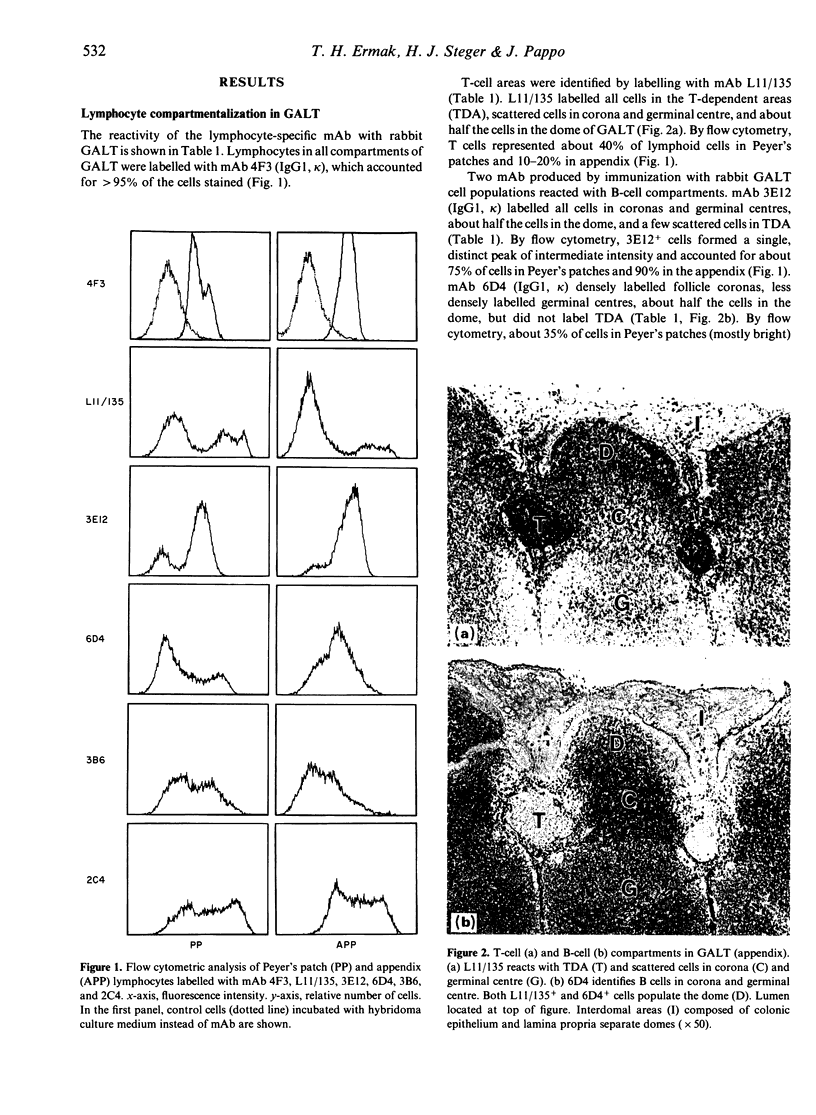
Abstract
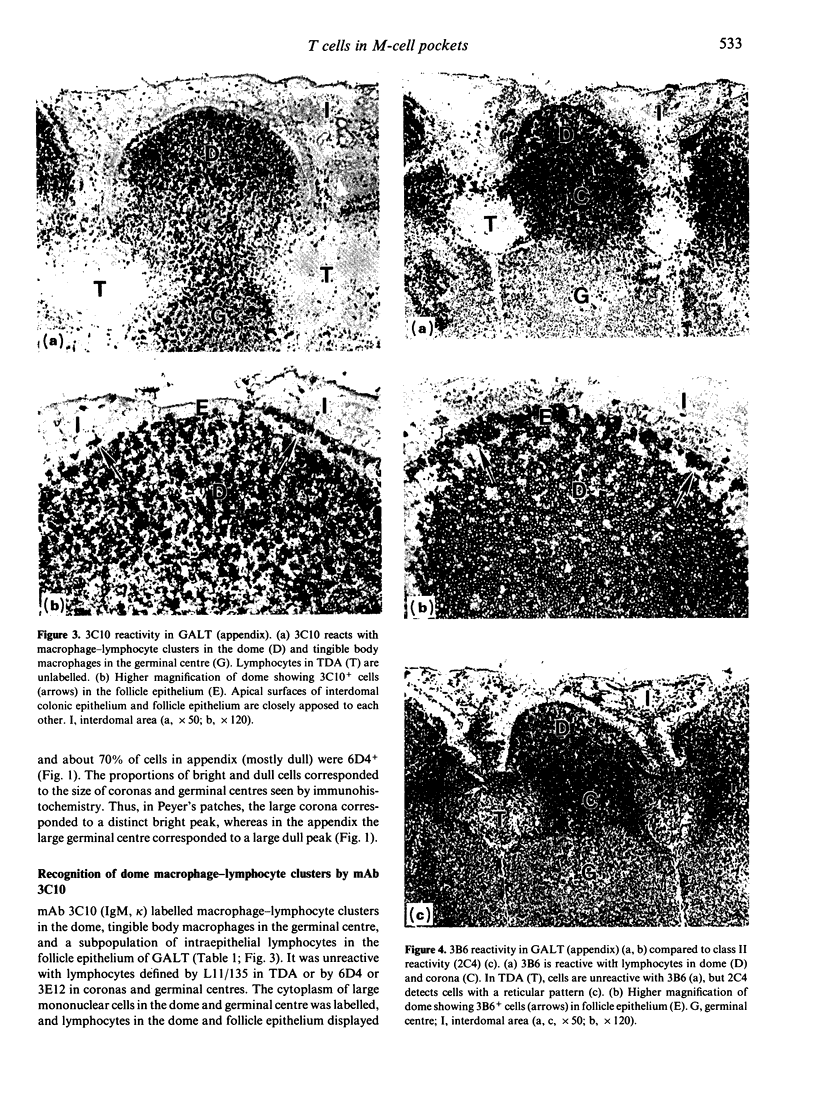
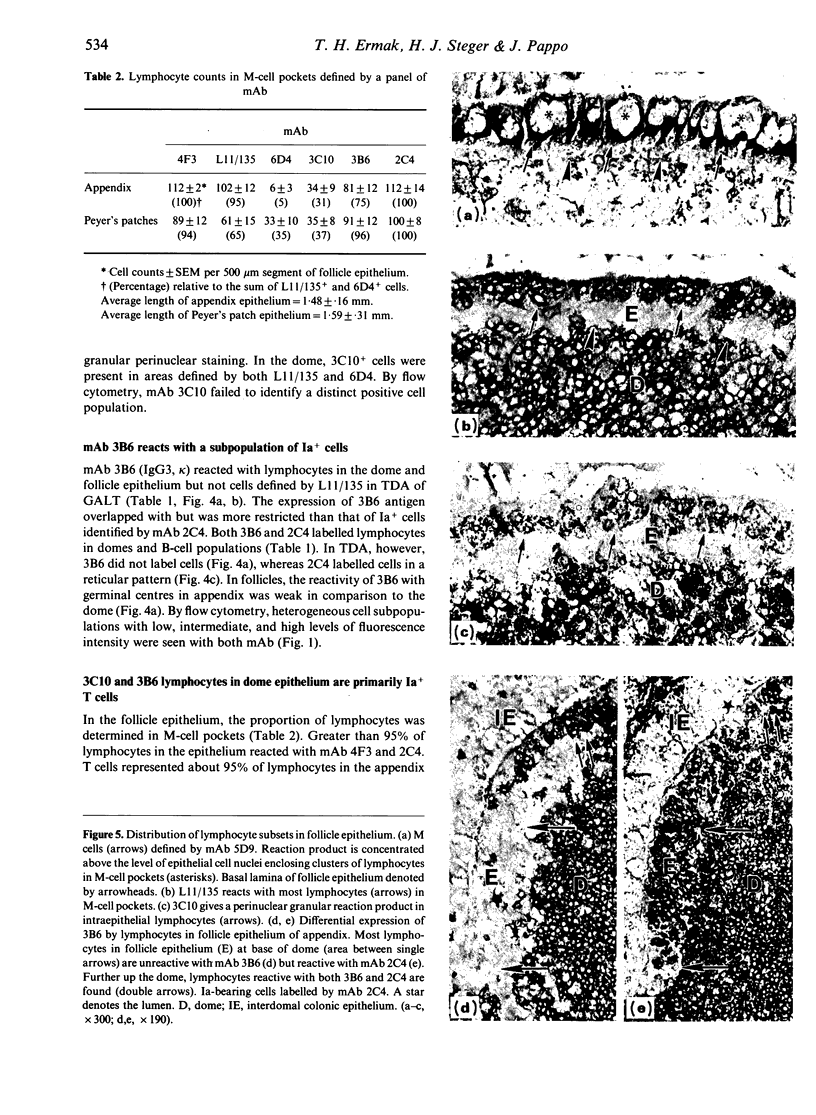
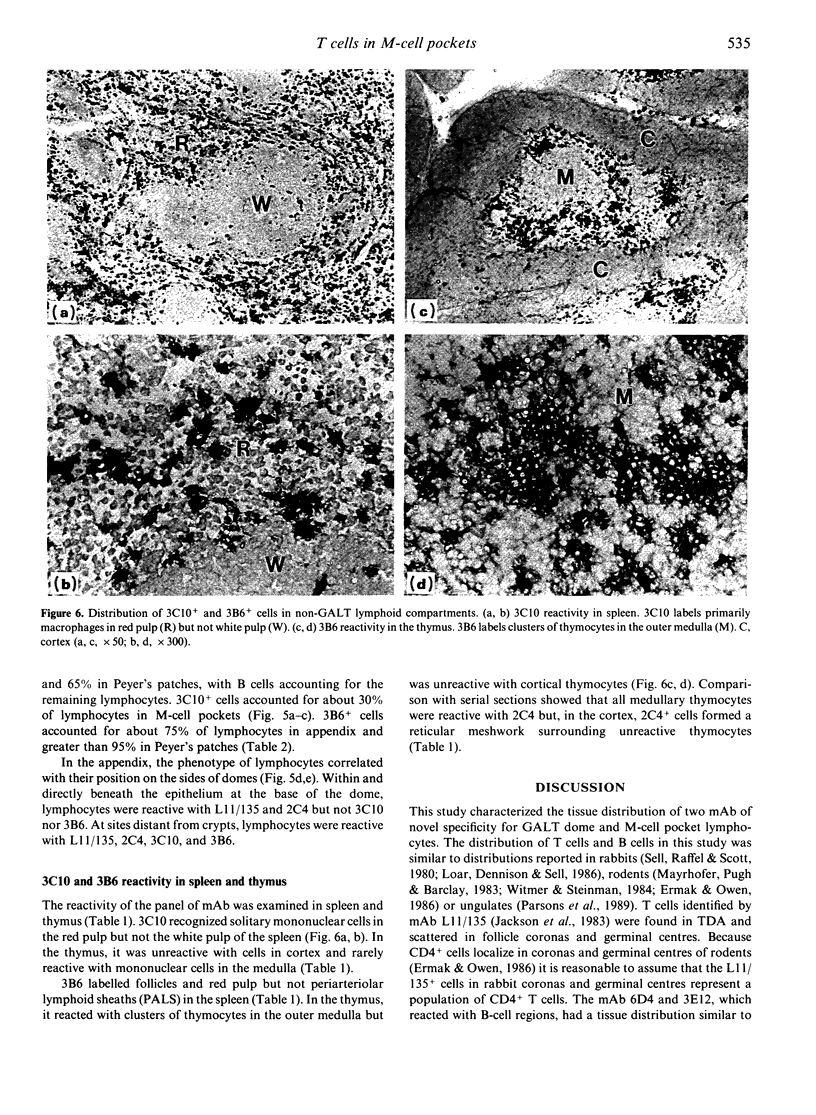
Follicle epithelium and domes of gut-associated lymphoid tissue (GALT) contain populations of lymphocytes which first contact antigen taken up from the intestine. In order to study the association of lymphocytes with M cells in follicle epithelium, monoclonal antibodies (mAb) were generated by immunizing BALB/c mice with lymphocytes populating GALT domes from NZW rabbits, and their specificity was assessed by immunohistochemistry and flow cytometry. mAb 3C10 (IgM) and 3B6 (IgG3) recognized subpopulations of intraepithelial lymphocytes associated with M cells. mAb 3C10 also identified macrophage-lymphocyte clusters in domes and tangible body macrophages in germinal centres of GALT but did not react with cells in T-dependent areas (TDA) or B cells in follicles. mAb 3B6 recognized lymphocytes in domes and B cells in follicles but not T cells in TDA of GALT. The distribution of 3B6+ cells overlapped with, but was more restricted than, that of Ia+ cells. Analysis of lymphocytes in follicle epithelium showed that greater than 95% of lymphocytes associated with M cells were Ia+. T cells represented approximately 95% of intraepithelial lymphocytes in the appendix and approximately 65% in Peyer's patches. A majority of intraepithelial lymphocytes was recognized by mAb 3B6, but mAb 3C10 identified only approximately 30%. Because neither 3C10 nor 3B6 recognized lymphocytes in TDA of GALT, these results indicate that most lymphocytes associated with M cells are a distinct phenotype of Ia+ T cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barg M., Draper L. R. Migration of thymus cells to the developing gut-associated lymphoid tissues of the young rabbit. Cell Immunol. 1975 Dec;20(2):177–186. doi: 10.1016/0008-8749(75)90095-7. [DOI] [PubMed] [Google Scholar]
- Bhalla D. K., Owen R. L. Cell renewal and migration in lymphoid follicles of Peyer's patches and cecum--an autoradiographic study in mice. Gastroenterology. 1982 Feb;82(2):232–242. [PubMed] [Google Scholar]
- Bhalla D. K., Owen R. L. Migration of B and T lymphocytes to M cells in Peyer's patch follicle epithelium: an autoradiographic and immunocytochemical study in mice. Cell Immunol. 1983 Oct 1;81(1):105–117. doi: 10.1016/0008-8749(83)90216-2. [DOI] [PubMed] [Google Scholar]
- Bockman D. E., Cooper M. D. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer's patches. An electron microscopic study. Am J Anat. 1973 Apr;136(4):455–477. doi: 10.1002/aja.1001360406. [DOI] [PubMed] [Google Scholar]
- Butcher E. C., Rouse R. V., Coffman R. L., Nottenburg C. N., Hardy R. R., Weissman I. L. Surface phenotype of Peyer's patch germinal center cells: implications for the role of germinal centers in B cell differentiation. J Immunol. 1982 Dec;129(6):2698–2707. [PubMed] [Google Scholar]
- Bye W. A., Allan C. H., Trier J. S. Structure, distribution, and origin of M cells in Peyer's patches of mouse ileum. Gastroenterology. 1984 May;86(5 Pt 1):789–801. [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A. Ontogenetic development of T- and B-lymphocytes and non-lymphoid cells in the white pulp of the rat spleen. Cell Tissue Res. 1983;229(2):351–363. doi: 10.1007/BF00214978. [DOI] [PubMed] [Google Scholar]
- Dobbins W. O., 3rd Human intestinal intraepithelial lymphocytes. Gut. 1986 Aug;27(8):972–985. doi: 10.1136/gut.27.8.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermak T. H., Owen R. L. Differential distribution of lymphocytes and accessory cells in mouse Peyer's patches. Anat Rec. 1986 Jun;215(2):144–152. doi: 10.1002/ar.1092150208. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., McCormick J. N., Goodman J. R., Yoffey J. M., Fudenberg H. H. Peyer's patches: morphologic studies. Cell Immunol. 1970 Nov;1(5):500–520. doi: 10.1016/0008-8749(70)90038-9. [DOI] [PubMed] [Google Scholar]
- Jackson S., Chused T. M., Wilkinson J. M., Leiserson W. M., Kindt T. J. Differentiation antigens identify subpopulations of rabbit T and B lymphocytes. Definition by flow cytometry. J Exp Med. 1983 Jan 1;157(1):34–46. doi: 10.1084/jem.157.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lause D. B., Bockman D. E. Heterogeneity, position, and functional capability of the macrophages in Peyer's patches. Cell Tissue Res. 1981;218(3):557–566. doi: 10.1007/BF00210115. [DOI] [PubMed] [Google Scholar]
- LeFevre M. E., Hammer R., Joel D. D. Macrophages of the mammalian small intestine: a review. J Reticuloendothel Soc. 1979 Nov;26(5):553–573. [PubMed] [Google Scholar]
- Loar L. D., Dennison D., Sell S. Production and characterization of monoclonal antibodies to rabbit lymphocyte subpopulations. I. Tissue immunofluorescence and flow cytometric analysis. J Immunol. 1986 Nov 1;137(9):2784–2790. [PubMed] [Google Scholar]
- Marcial M. A., Madara J. L. Cryptosporidium: cellular localization, structural analysis of absorptive cell-parasite membrane-membrane interactions in guinea pigs, and suggestion of protozoan transport by M cells. Gastroenterology. 1986 Mar;90(3):583–594. doi: 10.1016/0016-5085(86)91112-1. [DOI] [PubMed] [Google Scholar]
- Mayrhofer G., Pugh C. W., Barclay A. N. The distribution, ontogeny and origin in the rat of Ia-positive cells with dendritic morphology and of Ia antigen in epithelia, with special reference to the intestine. Eur J Immunol. 1983 Feb;13(2):112–122. doi: 10.1002/eji.1830130206. [DOI] [PubMed] [Google Scholar]
- McClugage S. G., Low F. N., Zimny M. L. Porosity of the basement membrane overlying Peyer's patches in rats and monkeys. Gastroenterology. 1986 Nov;91(5):1128–1133. doi: 10.1016/s0016-5085(86)80007-5. [DOI] [PubMed] [Google Scholar]
- Neutra M. R., Phillips T. L., Mayer E. L., Fishkind D. J. Transport of membrane-bound macromolecules by M cells in follicle-associated epithelium of rabbit Peyer's patch. Cell Tissue Res. 1987 Mar;247(3):537–546. doi: 10.1007/BF00215747. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Jones A. L. Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974 Feb;66(2):189–203. [PubMed] [Google Scholar]
- Owen R. L., Pierce N. F., Apple R. T., Cray W. C., Jr M cell transport of Vibrio cholerae from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986 Jun;153(6):1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- Owen R. L. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology. 1977 Mar;72(3):440–451. [PubMed] [Google Scholar]
- Pappo J., Ermak T. H. Uptake and translocation of fluorescent latex particles by rabbit Peyer's patch follicle epithelium: a quantitative model for M cell uptake. Clin Exp Immunol. 1989 Apr;76(1):144–148. [PMC free article] [PubMed] [Google Scholar]
- Pappo J. Generation and characterization of monoclonal antibodies recognizing follicle epithelial M cells in rabbit gut-associated lymphoid tissues. Cell Immunol. 1989 Apr 15;120(1):31–41. doi: 10.1016/0008-8749(89)90172-x. [DOI] [PubMed] [Google Scholar]
- Pappo J., Owen R. L. Absence of secretory component expression by epithelial cells overlying rabbit gut-associated lymphoid tissue. Gastroenterology. 1988 Nov;95(5):1173–1177. doi: 10.1016/0016-5085(88)90347-2. [DOI] [PubMed] [Google Scholar]
- Pappo J., Steger H. J., Owen R. L. Differential adherence of epithelium overlying gut-associated lymphoid tissue. An ultrastructural study. Lab Invest. 1988 Jun;58(6):692–697. [PubMed] [Google Scholar]
- Parsons K. R., Howard C. J., Jones B. V., Sopp P. Investigation of bovine gut associated lymphoid tissue (GALT) using monoclonal antibodies against bovine lymphocytes. Vet Pathol. 1989 Sep;26(5):396–408. doi: 10.1177/030098588902600505. [DOI] [PubMed] [Google Scholar]
- Sell S., Raffel C., Scott C. B. Tissue localization of T and B lymphocytes in lagomorphs: anatomical evidence for a major role of the gastrointestinal associated lymphoid tissue in generation of lymphocytes in the adult. Dev Comp Immunol. 1980 Spring;4(2):355–366. doi: 10.1016/s0145-305x(80)80038-3. [DOI] [PubMed] [Google Scholar]
- Takagaki Y., DeCloux A., Bonneville M., Tonegawa S. Diversity of gamma delta T-cell receptors on murine intestinal intra-epithelial lymphocytes. Nature. 1989 Jun 29;339(6227):712–714. doi: 10.1038/339712a0. [DOI] [PubMed] [Google Scholar]
- Van Ewijk W. Immunohistology of lymphoid and non-lymphoid cells in the thymus in relation to T lymphocyte differentiation. Am J Anat. 1984 Jul;170(3):311–330. doi: 10.1002/aja.1001700307. [DOI] [PubMed] [Google Scholar]
- Witmer M. D., Steinman R. M. The anatomy of peripheral lymphoid organs with emphasis on accessory cells: light-microscopic immunocytochemical studies of mouse spleen, lymph node, and Peyer's patch. Am J Anat. 1984 Jul;170(3):465–481. doi: 10.1002/aja.1001700318. [DOI] [PubMed] [Google Scholar]
- Wolf J. L., Kauffman R. S., Finberg R., Dambrauskas R., Fields B. N., Trier J. S. Determinants of reovirus interaction with the intestinal M cells and absorptive cells of murine intestine. Gastroenterology. 1983 Aug;85(2):291–300. [PubMed] [Google Scholar]