Abstract
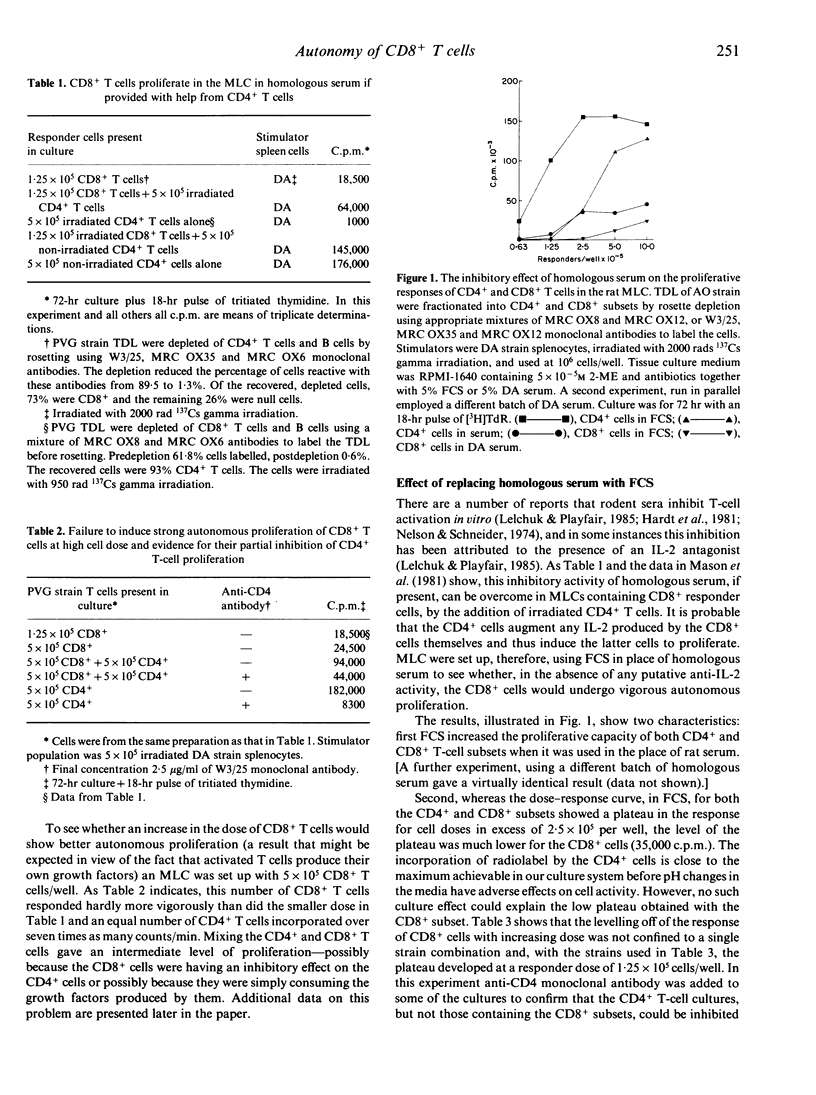
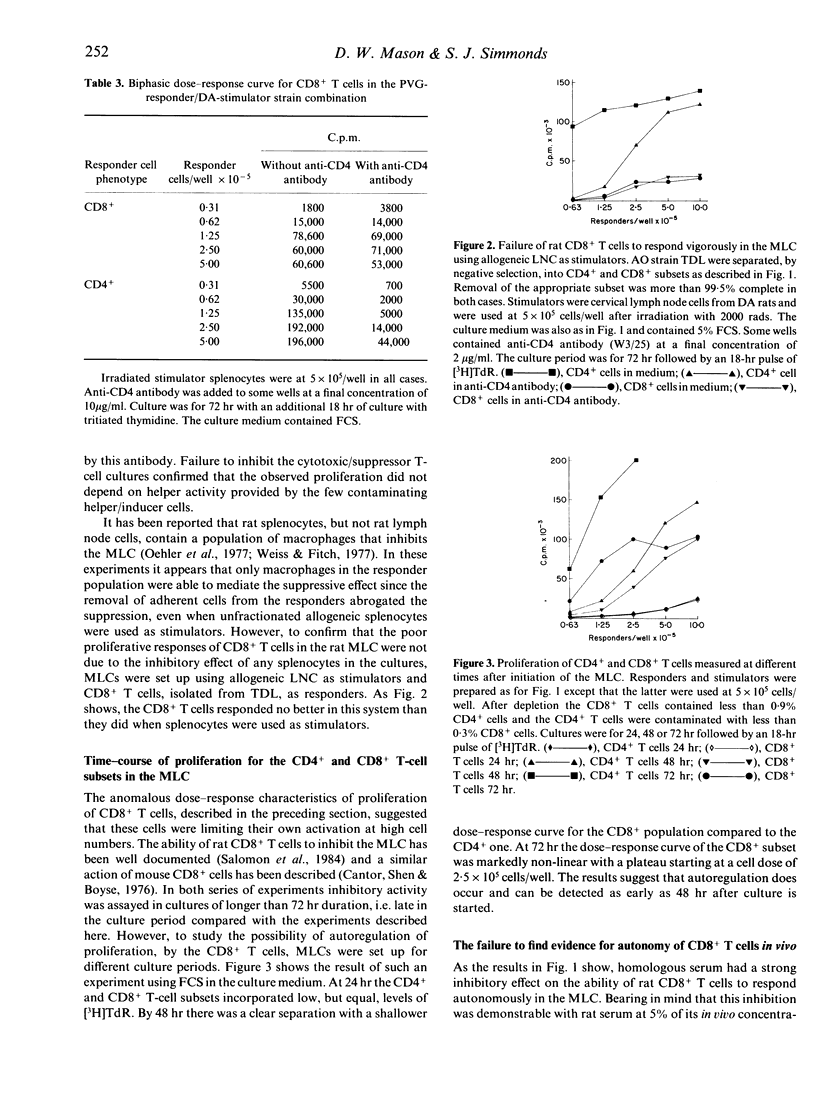
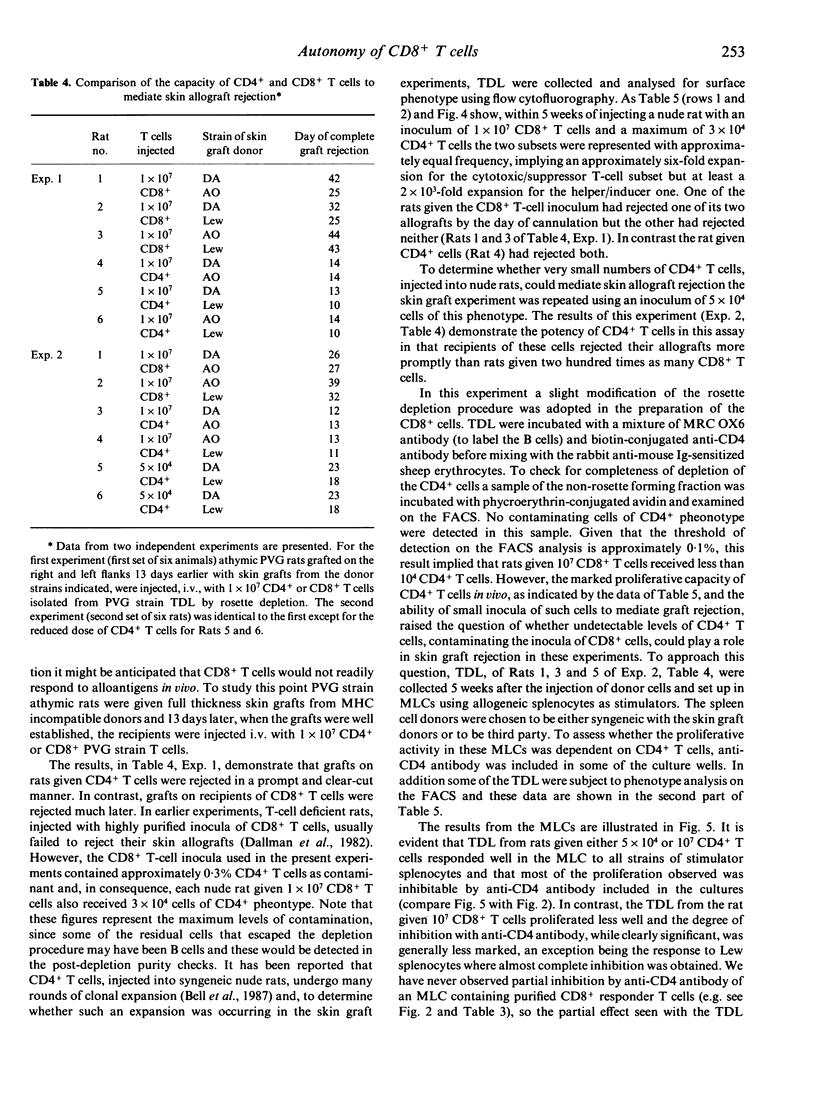
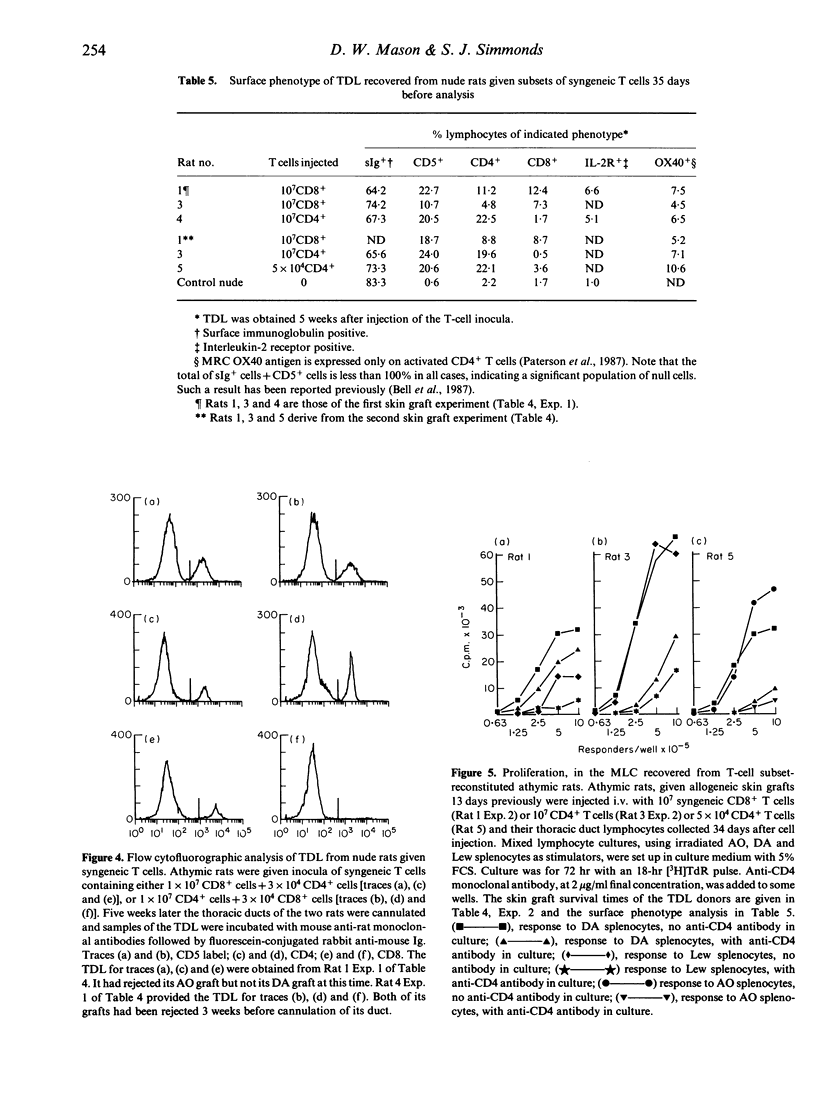
Experiments have been carried out in vitro and in vivo to determine to what extent CD8+ T cells in the rat can function independently of any helper activity from CD4+ cells. We have identified the culture conditions required for the autonomous proliferation of CD8+ T cells in the rat mixed leucocyte culture (MLC) and in particular have studied both the kinetics of the response and the effect of replacing the homologous serum, used in our previous MLC experiments, with fetal calf serum (FCS). The results obtained using FCS show that, early in the MLC, CD8+ T-cells proliferate at a comparable rate to the CD4+ subset but that, within 48-72 hr, the proliferation rate of the CD8+ cells ceases to increase with time. In contrast, the proliferation of the CD4+ T cells appears to be limited only by the exhaustion of the culture medium. The results also show that the proliferative responses of both CD4+ and CD8+ T cells are inhibited in homologous serum but that it is the CD8+ subset that is more affected. When CD8+ T cells, in homologous serum, are co-cultured with irradiated CD4+ T cells the proliferative activity is increased, indicating that the helper activity of the CD4+ T cells can over-ride the inhibitory effect of the serum. In vivo we have compared the abilities of injected CD4+ and CD8+ T cells to mediate rejection of skin allografts on nude rats. Grafts were rejected more rapidly on recipients of low doses of CD4+ T cells than on rats given 200 times as many CD8+ T cells. Thoracic duct lymphocytes (TDL) obtained 5 weeks after CD8+ T-cell injection always contained a population of CD4+ T cells, even when the injected CD8+ T-cell inoculum contained less than 0.1% CD4+ cells as contaminants. Evidence was obtained that these CD4+ T cells found in TDL displayed alloreactivity in MLC. Further, the intentional injection of very low doses of CD4+ cells led, after 5 weeks, to frequencies of CD4+ T cells, in thoracic duct lymph, equal to that obtained by the injection of 200 times as many cells of the same phenotype. It appears that, in T-cell-deficient rats, CD4+ cells can expand over 2000 times in a few weeks. Such expansion may explain the relatively slow rejection of skin allografts observed in these experiments when nude rats were injected with putatively pure populations of CD8+ T cells.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach F. H., Grillot-Courvalin C., Kuperman O. J., Sollinger H. W., Hayes C., Sondel P. M., Alter B. J., Bach M. L. Antigenic requirements for triggering of cytotoxic T lymphocytes. Immunol Rev. 1977;35:76–96. doi: 10.1111/j.1600-065x.1977.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Bell E. B., Sparshott S. M., Drayson M. T., Ford W. L. The stable and permanent expansion of functional T lymphocytes in athymic nude rats after a single injection of mature T cells. J Immunol. 1987 Sep 1;139(5):1379–1384. [PubMed] [Google Scholar]
- Born W., Wekerle H. Lympho-stromal interactions in the thymus: medullary thymocytes react with I-A determinants on autochthonous thymic stimulator cells. Eur J Immunol. 1982 Jan;12(1):51–59. doi: 10.1002/eji.1830120111. [DOI] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T lymphocytes bearing different Ly antigens. II. Cooperation between subclasses of Ly+ cells in the generation of killer activity. J Exp Med. 1975 Jun 1;141(6):1390–1399. doi: 10.1084/jem.141.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Shen F. W., Boyse E. A. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. A., Robins R. A., Baldwin R. W. Rat lymphocyte subsets: cellular requirements for the generation of T-cell growth factor. Cell Immunol. 1982 Jul 1;70(2):367–372. doi: 10.1016/0008-8749(82)90338-0. [DOI] [PubMed] [Google Scholar]
- Damle N. K., Mohagheghpour N., Hansen J. A., Engleman E. G. Alloantigen-specific cytotoxic and suppressor T lymphocytes are derived from phenotypically distinct precursors. J Immunol. 1983 Nov;131(5):2296–2300. [PubMed] [Google Scholar]
- GOWANS J. L. The fate of parental strain small lymphocytes in F1 hybrid rats. Ann N Y Acad Sci. 1962 Oct 24;99:432–455. doi: 10.1111/j.1749-6632.1962.tb45326.x. [DOI] [PubMed] [Google Scholar]
- Hall B. M., de Saxe I., Dorsch S. E. The cellular basis of allograft rejection in vivo. III. Restoration of first-set rejection of heart grafts by T helper cells in irradiated rats. Transplantation. 1983 Dec;36(6):700–705. doi: 10.1097/00007890-198336060-00023. [DOI] [PubMed] [Google Scholar]
- Hardt C., Röllinghoff M., Pfizenmaier K., Mosmann H., Wagner H. Lyt-23+ cyclophosphamide-sensitive T cells regulate the activity of an interleukin 2 inhibitor in vivo. J Exp Med. 1981 Aug 1;154(2):262–274. doi: 10.1084/jem.154.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Young J. W., Steinman R. M. Direct activation of CD8+ cytotoxic T lymphocytes by dendritic cells. J Exp Med. 1987 Jul 1;166(1):182–194. doi: 10.1084/jem.166.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelchuk R., Playfair J. H. Serum IL-2 inhibitor in mice. I. Increase during infection. Immunology. 1985 Sep;56(1):113–118. [PMC free article] [PubMed] [Google Scholar]
- Loop S. M., Bernstein I. D., Wright P. W. T cell synergy in the rat: serologic characterization of T cell subsets. J Immunol. 1980 Sep;125(3):1237–1239. [PubMed] [Google Scholar]
- Mason D. W. Subsets of T cells in the rat mediating lethal graft versus-host disease. Transplantation. 1981 Sep;32(3):222–226. doi: 10.1097/00007890-198109000-00008. [DOI] [PubMed] [Google Scholar]
- Mason D. W. The possible role of class II major histocompatibility complex antigens in self tolerance. Scand J Immunol. 1985 May;21(5):397–400. doi: 10.1111/j.1365-3083.1985.tb01824.x. [DOI] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Miller R. A., Stutman O. Use of positively selected Lyt-2+ mouse splenocytes to examine interleukin-2 secretion in responses to alloantigens and to TNP-modified syngeneic cells. Cell Immunol. 1982 Mar 15;68(1):114–127. doi: 10.1016/0008-8749(82)90094-6. [DOI] [PubMed] [Google Scholar]
- Mitchison N. A., O'Malley C. Three-cell-type clusters of T cells with antigen-presenting cells best explain the epitope linkage and noncognate requirements of the in vivo cytolytic response. Eur J Immunol. 1987 Nov;17(11):1579–1583. doi: 10.1002/eji.1830171109. [DOI] [PubMed] [Google Scholar]
- Nelson D. S., Shneider C. N. Effect of normal mouse serum on mouse lymphocyte transformation in vitro. Eur J Immunol. 1974 Feb;4(2):79–86. doi: 10.1002/eji.1830040205. [DOI] [PubMed] [Google Scholar]
- Oehler J. R., Herberman R. B., Campbell D. A., Jr, Djeu J. Y. Inhibition of rat mixed lymphocyte cultures by suppressor macrophages. Cell Immunol. 1977 Mar 15;29(2):238–250. doi: 10.1016/0008-8749(77)90319-7. [DOI] [PubMed] [Google Scholar]
- Paterson D. J., Jefferies W. A., Green J. R., Brandon M. R., Corthesy P., Puklavec M., Williams A. F. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987 Dec;24(12):1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. S., Mizuochi T., Singer A. Analysis of T-cell subsets in rejection of Kb mutant skin allografts differing at class I MHC. 1986 Aug 28-Sep 3Nature. 322(6082):829–831. doi: 10.1038/322829a0. [DOI] [PubMed] [Google Scholar]
- Salomon D. R., Cohen D. J., Williams J. M., Carpenter C. B. T cell synergy in the primary MLR: proliferative kinetics, effector cell generation, and IL 2 production. J Immunol. 1984 Dec;133(6):3075–3083. [PubMed] [Google Scholar]
- Singer A., Munitz T. I., Golding H., Rosenberg A. S., Mizuochi T. Recognition requirements for the activation, differentiation and function of T-helper cells specific for class I MHC alloantigens. Immunol Rev. 1987 Aug;98:143–170. doi: 10.1111/j.1600-065x.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Sopori M. L., Cohen D. A., Cherian S., Roszman T. L., Kaplan A. M. T-lymphocyte heterogeneity in the rat: separation of distinct rat T-lymphocyte populations which respond in syngeneic and allogeneic mixed lymphocyte reactions. Cell Immunol. 1984 Aug;87(1):295–303. doi: 10.1016/0008-8749(84)90153-9. [DOI] [PubMed] [Google Scholar]
- Sprent J., Schaefer M. Capacity of purified Lyt-2+ T cells to mount primary proliferative and cytotoxic responses to Ia- tumour cells. Nature. 1986 Aug 7;322(6079):541–544. doi: 10.1038/322541a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Schaefer M., Lo D., Korngold R. Properties of purified T cell subsets. II. In vivo responses to class I vs. class II H-2 differences. J Exp Med. 1986 Apr 1;163(4):998–1011. doi: 10.1084/jem.163.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Schaefer M. Properties of purified T cell subsets. I. In vitro responses to class I vs. class II H-2 alloantigens. J Exp Med. 1985 Dec 1;162(6):2068–2088. doi: 10.1084/jem.162.6.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Nussenzweig M. C. Dendritic cells: features and functions. Immunol Rev. 1980;53:127–147. doi: 10.1111/j.1600-065x.1980.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Wagner H., Röllinghoff M. T-T-cell interactions during the vitro cytotoxic allograft responses. I. Soluble products from activated Lyl+ T cells trigger autonomously antigen-primed Ly23+ T cells to cell proliferation and cytolytic activity. J Exp Med. 1978 Dec 1;148(6):1523–1538. doi: 10.1084/jem.148.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Fitch F. W. Macrophages suppress CTL generation in rat mixed leukocyte cultures. J Immunol. 1977 Aug;119(2):510–516. [PubMed] [Google Scholar]
- White R. A., Mason D. W., Williams A. F., Galfre G., Milstein C. T-lymphocyte heterogeneity in the rat: separation of functional subpopulations using a monoclonal antibody. J Exp Med. 1978 Sep 1;148(3):664–673. doi: 10.1084/jem.148.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]


