Abstract
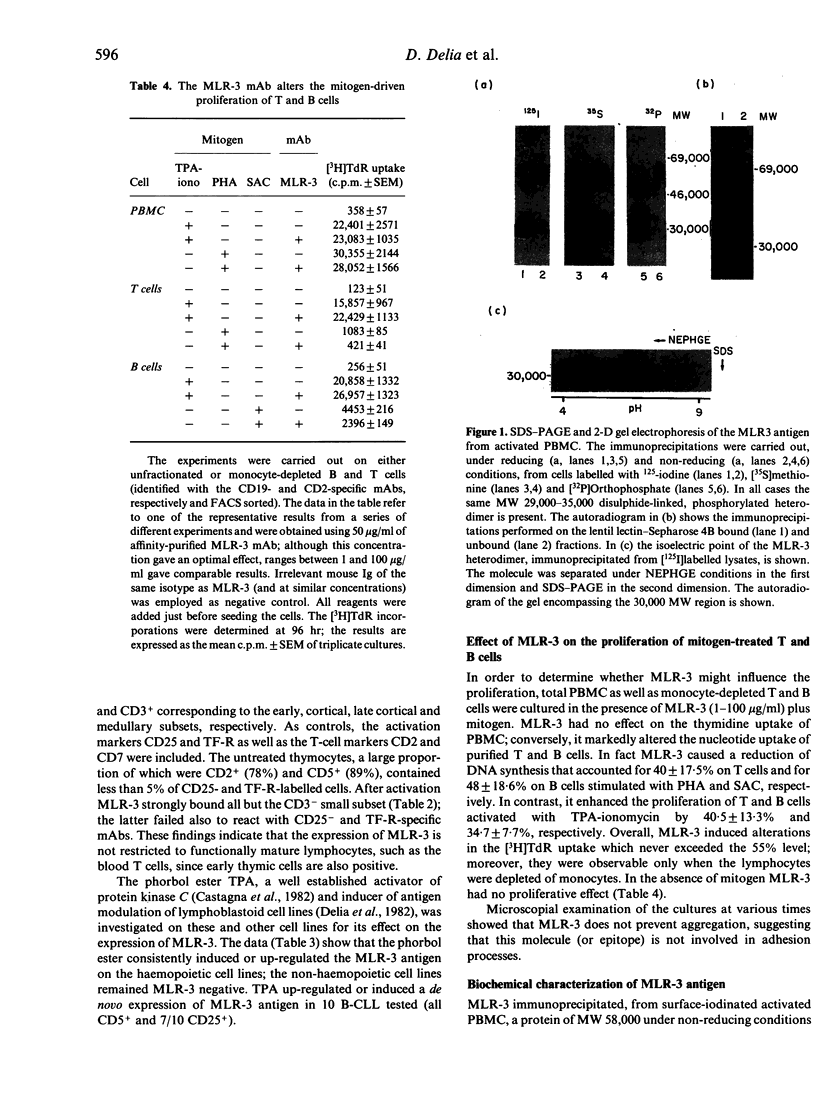
The MLR-3 monoclonal antibody reacts with activated but not with resting lymphocytes. We report that MLR-3 identifies an early activation molecule since its binding is detectable on T cells 1.5-2 hr after in vitro activation. Its expression, therefore, does not require DNA synthesis and precedes, by many hours, that of the receptors for interleukin-2 (IL-2R) and transferrin (TF-R). The MLR-3 antigen is also found on activated thymocytes (including the large early thymic CD3- subset) and B cells. The majority of T- and B-lymphoblastoid cell lines, as well as the myeloid and erythroid cell lines HL60, GM1 and K562, are MLR-3+; conversely, non-haemopoietic cell lines are MLR-3 negative. Seventy percent of B-cell chronic lymphocytic leukaemia and 15% of B non-Hodgkin's lymphomas (B-NHL) are MLR-3+. On tissue sections MLR-3 is reactive with epithelia, sweat glands, hair follicles and Henle's loop but not with vessels, connective, endothelium and many other tissues. In vitro studies show that MLR-3 (1-100 micrograms/ml) significantly alters the thymidine uptake of mitogen-treated lymphocytes:augmentation is found when T and B cells are induced with TPA-Ionomycin and reduction when induced with phytohaemoagglutinin (PHA) or Staphylococcus aureus Cowan strain 1 (SAC), respectively. On SDS-PAGE, MLR-3 immunoprecipitates a disulphide-linked heterodimer of MW 29,000-35,000: both subunits are glycosylated, phosphorylated and exhibit a pI of 4.1 and 5.0, respectively. Our data, particularly the in vitro results, suggest that the MRL-3 molecule could have an important role in the early hours of activation for the progression of resting lymphocytes into mitosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Corte G., Moretta L., Damiani G., Mingari M. C., Bargellesi A. Surface antigens specifically expressed by activated T cells in humans. Eur J Immunol. 1981 Feb;11(2):162–164. doi: 10.1002/eji.1830110220. [DOI] [PubMed] [Google Scholar]
- Cosulich M. E., Rubartelli A., Risso A., Cozzolino F., Bargellesi A. Functional characterization of an antigen involved in an early step of T-cell activation. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4205–4209. doi: 10.1073/pnas.84.12.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delia D., Greaves M. F., Newman R. A., Sutherland D. R., Minowada J., Kung P., Goldstein G. Modulation of T leukaemic cell phenotype with phorbol ester. Int J Cancer. 1982 Jan 15;29(1):23–31. doi: 10.1002/ijc.2910290106. [DOI] [PubMed] [Google Scholar]
- Delia D., Greaves M., Villa S., DeBraud F. Characterization of the response of human thymocytes and blood lymphocytes to the synergistic mitogenicity of 12-O-tetradecanoylphorbol-13-acetate (TPA)-ionomycin. Eur J Immunol. 1984 Aug;14(8):720–724. doi: 10.1002/eji.1830140809. [DOI] [PubMed] [Google Scholar]
- Dower S. K., Kronheim S. R., March C. J., Conlon P. J., Hopp T. P., Gillis S., Urdal D. L. Detection and characterization of high affinity plasma membrane receptors for human interleukin 1. J Exp Med. 1985 Aug 1;162(2):501–515. doi: 10.1084/jem.162.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durum S. K., Schmidt J. A., Oppenheim J. J. Interleukin 1: an immunological perspective. Annu Rev Immunol. 1985;3:263–287. doi: 10.1146/annurev.iy.03.040185.001403. [DOI] [PubMed] [Google Scholar]
- Hancock W. W., Muller W. A., Cotran R. S. Interleukin 2 receptors are expressed by alveolar macrophages during pulmonary sarcoidosis and are inducible by lymphokine treatment of normal human lung macrophages, blood monocytes, and monocyte cell lines. J Immunol. 1987 Jan 1;138(1):185–191. [PubMed] [Google Scholar]
- Hara T., Jung L. K., Bjorndahl J. M., Fu S. M. Human T cell activation. III. Rapid induction of a phosphorylated 28 kD/32 kD disulfide-linked early activation antigen (EA 1) by 12-o-tetradecanoyl phorbol-13-acetate, mitogens, and antigens. J Exp Med. 1986 Dec 1;164(6):1988–2005. doi: 10.1084/jem.164.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Jacobson J. G., Strominger J. L. Biochemical characterization of VLA-1 and VLA-2. Cell surface heterodimers on activated T cells. J Biol Chem. 1985 Dec 5;260(28):15246–15252. [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Greene W. C. Sequential expression of genes involved in human T lymphocyte growth and differentiation. J Exp Med. 1985 Jun 1;161(6):1593–1598. doi: 10.1084/jem.161.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Newman W., Fanning V. A., Rao P. E., Westberg E. F., Patten E. Early events in lymphocyte activation as defined by three new monoclonal antibodies. J Immunol. 1986 Dec 15;137(12):3702–3708. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pierotti M., DeLeo A. B., Pinter A., O'Donnell P. V., Hämmerling U., Fleissner E. The GIX antigen of murine leukemia virus: an analysis with monoclonal antibodies. Virology. 1981 Jul 30;112(2):450–460. doi: 10.1016/0042-6822(81)90292-0. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Alpers J. D., Nowell P. C., Hoover R. G. Sequential expression of protooncogenes during lectin-stimulated mitogenesis of normal human lymphocytes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3982–3986. doi: 10.1073/pnas.83.11.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Fritz M. E., Biddison W. E., Boutin B., Yarchoan R., Nelson D. L. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985 Nov;135(5):3172–3177. [PubMed] [Google Scholar]
- Suomalainen H. A. The monoclonal antibodies Trop-4 and 4F2 detect the same membrane antigen that is expressed at an early stage of lymphocyte activation and is retained on secondary lymphocytes. J Immunol. 1986 Jul 15;137(2):422–427. [PubMed] [Google Scholar]
- Takada Y., Huang C., Hemler M. E. Fibronectin receptor structures in the VLA family of heterodimers. Nature. 1987 Apr 9;326(6113):607–609. doi: 10.1038/326607a0. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Verbi W., Greaves M. F., Schneider C., Koubek K., Janossy G., Stein H., Kung P., Goldstein G. Monoclonal antibodies OKT 11 and OKT 11A have pan-T reactivity and block sheep erythrocyte "receptors". Eur J Immunol. 1982 Jan;12(1):81–86. doi: 10.1002/eji.1830120115. [DOI] [PubMed] [Google Scholar]
- Vodinelich L., Tax W., Bai Y., Pegram S., Capel P., Greaves M. F. A monoclonal antibody (WT1) for detecting leukemias of T-cell precursors (T-ALL). Blood. 1983 Nov;62(5):1108–1113. [PubMed] [Google Scholar]
- Walker L., Guy G., Brown G., Rowe M., Milner A. E., Gordon J. Control of human B-lymphocyte replication. I. Characterization of novel activation states that precede the entry of G0 B cells into cycle. Immunology. 1986 Aug;58(4):583–589. [PMC free article] [PubMed] [Google Scholar]



