Abstract
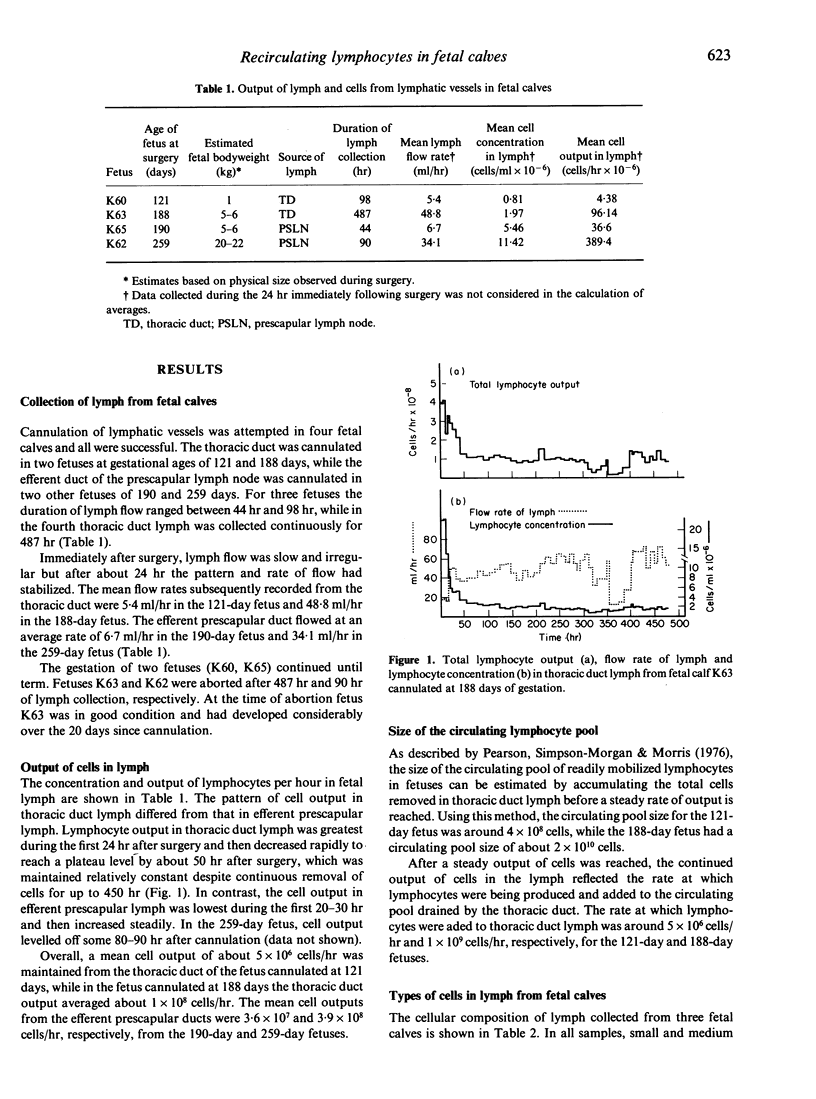
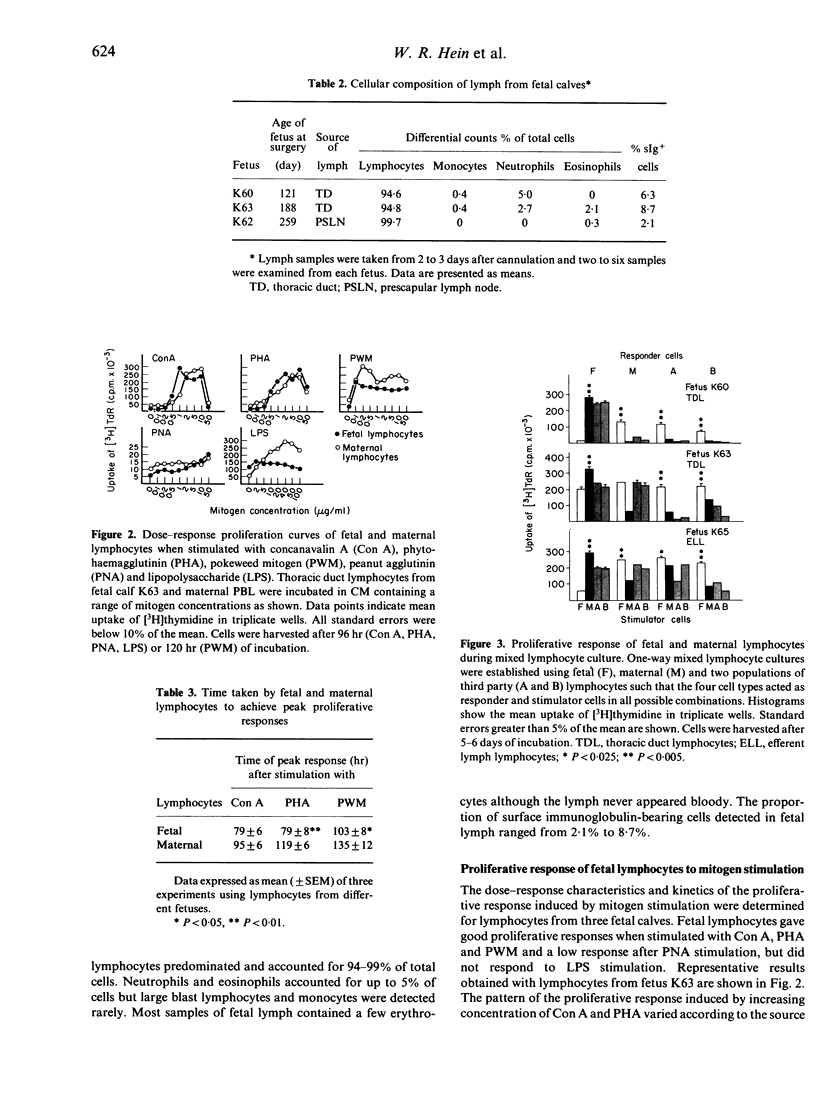
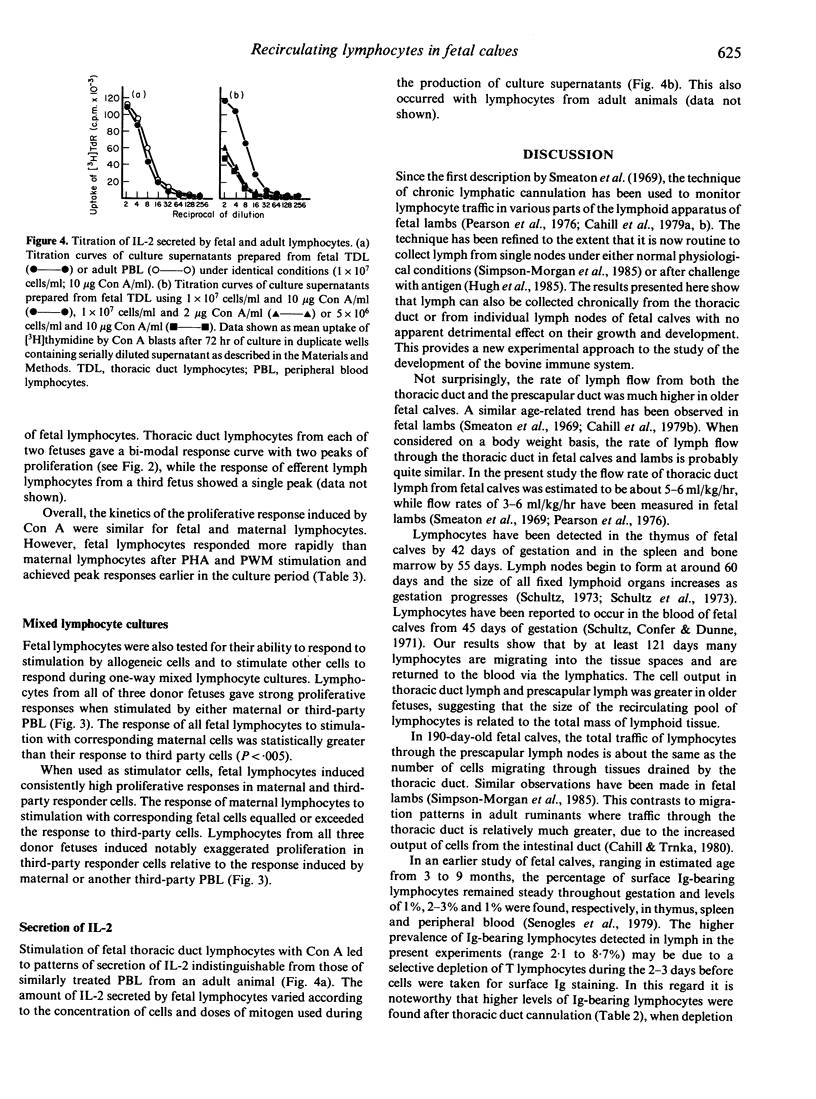
The thoracic duct or efferent prescapular duct was cannulated in four fetal calves aged 121-259 days post-conception. The duration of lymph flow ranged from 2 to 20 days and the mean flow rates sustained over these collection periods varied from 5.4 to 48.8 ml/hr. Lymphocyte output ranged from 4.4 x 10(6) cells/hr in thoracic duct lymph from a 121-day fetus to 3.9 x 10(8) cells/hr in efferent prescapular lymph from a 259-day fetus. The circulating lymphocyte pool in fetal calves of about 120 and 190 days gestational age was calculated to contain, respectively, 4 x 10(8) cells and 2 x 10(10) cells. The proportion of lymphocytes bearing surface immunoglobulin detected in fetal lymph ranged from 2.1% to 8.7%. Recirculating lymphocytes from fetal calves produced strong proliferative responses when stimulated by T-cell mitogens but responded poorly to B-cell mitogens. Fetal lymphocytes also responded to stimulation by allogeneic cells and stimulated other cells to proliferate during mixed lymphocyte culture. When stimulated with Con A, fetal lymphocytes secreted IL-2 to a degree that was indistinguishable from the secretory behaviour of lymphocytes from adult animals. The results presented in this paper show that chronic lymphatic fistulae can be established successfully in fetal calves to give access to recirculating lymphocytes. This provides a new experimental approach for studying the development of the bovine immune system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cahill R. N., Poskitt D. C., Hay J. B., Heron I., Trnka Z. The migration of lymphocytes in the fetal lamb. Eur J Immunol. 1979 Mar;9(3):251–253. doi: 10.1002/eji.1830090315. [DOI] [PubMed] [Google Scholar]
- Cahill R. N., Poskitt D. C., Heron I., Trnka Z. Collection of lymph from single lymph nodes and the intestines of fetal lambs in utero. Int Arch Allergy Appl Immunol. 1979;59(1):117–120. doi: 10.1159/000232248. [DOI] [PubMed] [Google Scholar]
- Enright F. M., Walker J. V., Jeffers G., Deyoe B. L. Cellular and humoral responses of Brucella abortus-infected bovine fetuses. Am J Vet Res. 1984 Mar;45(3):424–430. [PubMed] [Google Scholar]
- Hein W. R., Shelton J. N., Simpson-Morgan M. W., Seamark R. F., Morris B. Flow and composition of lymph from the ovary and uterus of cows during pregnancy. J Reprod Fertil. 1988 May;83(1):309–323. doi: 10.1530/jrf.0.0830309. [DOI] [PubMed] [Google Scholar]
- Hugh A. R., Trevella W., Simpson-Morgan M. W., Morris B. The lymph-borne response of foetal lamb lymph nodes to challenge with Brucella abortus in utero. Aust J Exp Biol Med Sci. 1985 Aug;63(Pt 4):381–395. doi: 10.1038/icb.1985.44. [DOI] [PubMed] [Google Scholar]
- Kasakura S. A blastogenic factor in unidirectional mixed cultures with x-irradiated cells. Transplantation. 1971 Feb;11(2):117–121. doi: 10.1097/00007890-197102000-00002. [DOI] [PubMed] [Google Scholar]
- Lafferty K. J., Prowse S. J., Al-Adra A., Warren H. S., Vasalli J., Reich E. An improved assay for interleukin 2 (lymphocyte growth factor) produced by mitogen-activated lymphocytes. Aust J Exp Biol Med Sci. 1980 Dec;58(6):533–544. doi: 10.1038/icb.1980.55. [DOI] [PubMed] [Google Scholar]
- Liggitt H. D., DeMartini J. C., Pearson L. D. Immunologic responses of the bovine fetus to parvovirus infection. Am J Vet Res. 1982 Aug;43(8):1355–1359. [PubMed] [Google Scholar]
- MacLachlan N. J., Schore C. E., Osburn B. I. Lymphocyte blastogenesis in bluetongue virus or Mycobacterium bovis-inoculated bovine fetuses. Vet Immunol Immunopathol. 1984 Aug;7(1):11–18. doi: 10.1016/0165-2427(84)90023-0. [DOI] [PubMed] [Google Scholar]
- Miller-Edge M., Splitter G. A. Bovine interleukin 2 (IL-2) production and activity on bovine and murine cell lines. Vet Immunol Immunopathol. 1984 Sep;7(2):119–130. doi: 10.1016/0165-2427(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Miyasaka M., McCullagh P. Immunological responsiveness of maternal and foetal lymphocytes during normal pregnancy in the ewe. J Reprod Immunol. 1981 May;3(1):15–27. doi: 10.1016/0165-0378(81)90025-5. [DOI] [PubMed] [Google Scholar]
- Miyasaka M., McCullagh P. The response of the foetal lamb to maternal lymphocytes. J Reprod Immunol. 1982 Aug;4(4):217–230. doi: 10.1016/0165-0378(82)90028-6. [DOI] [PubMed] [Google Scholar]
- Namen A. E., Magnuson J. A. Production and characterization of bovine interleukin-2. Immunology. 1984 Jul;52(3):469–475. [PMC free article] [PubMed] [Google Scholar]
- Oldham G., Williams L. Interleukin 2 (IL-2) production by mitogen stimulated bovine peripheral blood lymphocytes and its assay. Vet Immunol Immunopathol. 1984 Oct;7(3-4):201–212. doi: 10.1016/0165-2427(84)90079-5. [DOI] [PubMed] [Google Scholar]
- Pearson L. D., Simpson-Morgan M. W., Morris B. Lymphopoiesis and lymphocyte recirculation in the sheep fetus. J Exp Med. 1976 Jan 1;143(1):167–186. doi: 10.1084/jem.143.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw H. W., Eckblad W. P., Everson D. O., Tassinari P. D., Amos D. Ontogeny of immunocompetence in cattle: evaluation of phytomitogen-induced in vitro bovine fetal lymphocyte blastogenesis, using a whole blood culture technique. Am J Vet Res. 1977 Aug;38(8):1141–1150. [PubMed] [Google Scholar]
- Schultz R. D., Confer F., Dunne H. W. Occurrence of blood cells and serum proteins in bovine fetuses and calves. Can J Comp Med. 1971 Apr;35(2):93–98. [PMC free article] [PubMed] [Google Scholar]
- Schultz R. D., Dunne H. W., Heist C. E. Ontogeny of the bovine immune response. Infect Immun. 1973 Jun;7(6):981–991. doi: 10.1128/iai.7.6.981-991.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senogles D. R., Paul P. S., Johnson D. W., Muscoplat C. C. Ontogeny of T cells, B cells and monocytes in the bovine foetus. Clin Exp Immunol. 1979 May;36(2):299–303. [PMC free article] [PubMed] [Google Scholar]
- Simpson-Morgan M. W., Trevella W., Hugh A. R., McClure S. J., Morris B. The long-term collection of lymph from single lymph nodes of foetal lambs in utero. Aust J Exp Biol Med Sci. 1985 Aug;63(Pt 4):397–409. doi: 10.1038/icb.1985.45. [DOI] [PubMed] [Google Scholar]
- Smeaton T. C., Cole G. J., Simpson-Morgan M. W., Morris B. Techniques for the long-term collection of lymph from the unanaesthetized foetal lamb in utero. Aust J Exp Biol Med Sci. 1969 Oct;47(5):565–572. doi: 10.1038/icb.1969.150. [DOI] [PubMed] [Google Scholar]


