Abstract
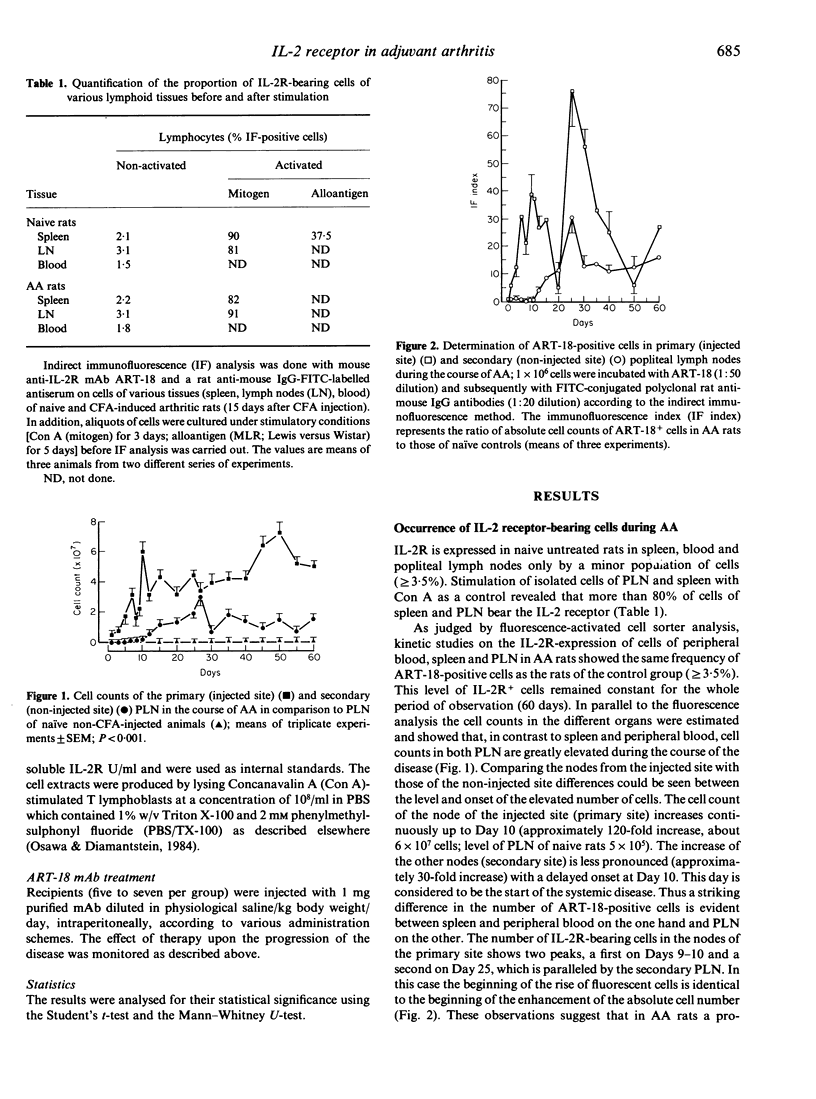
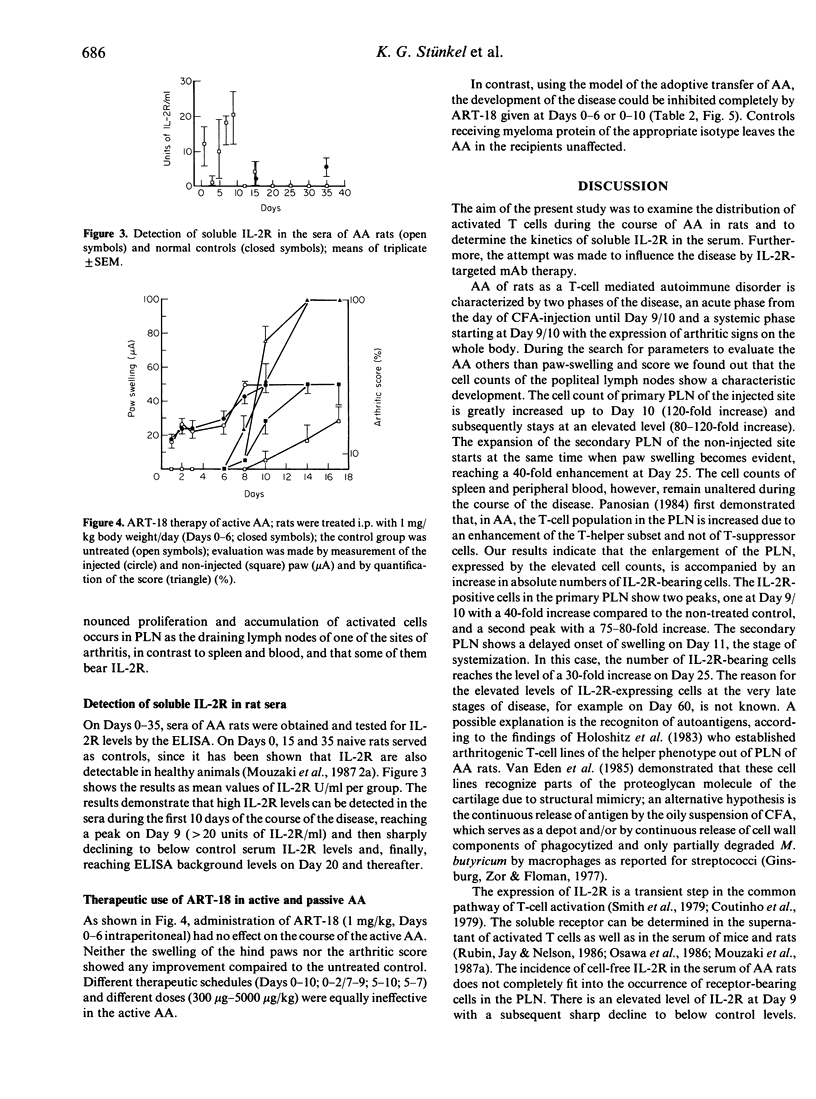
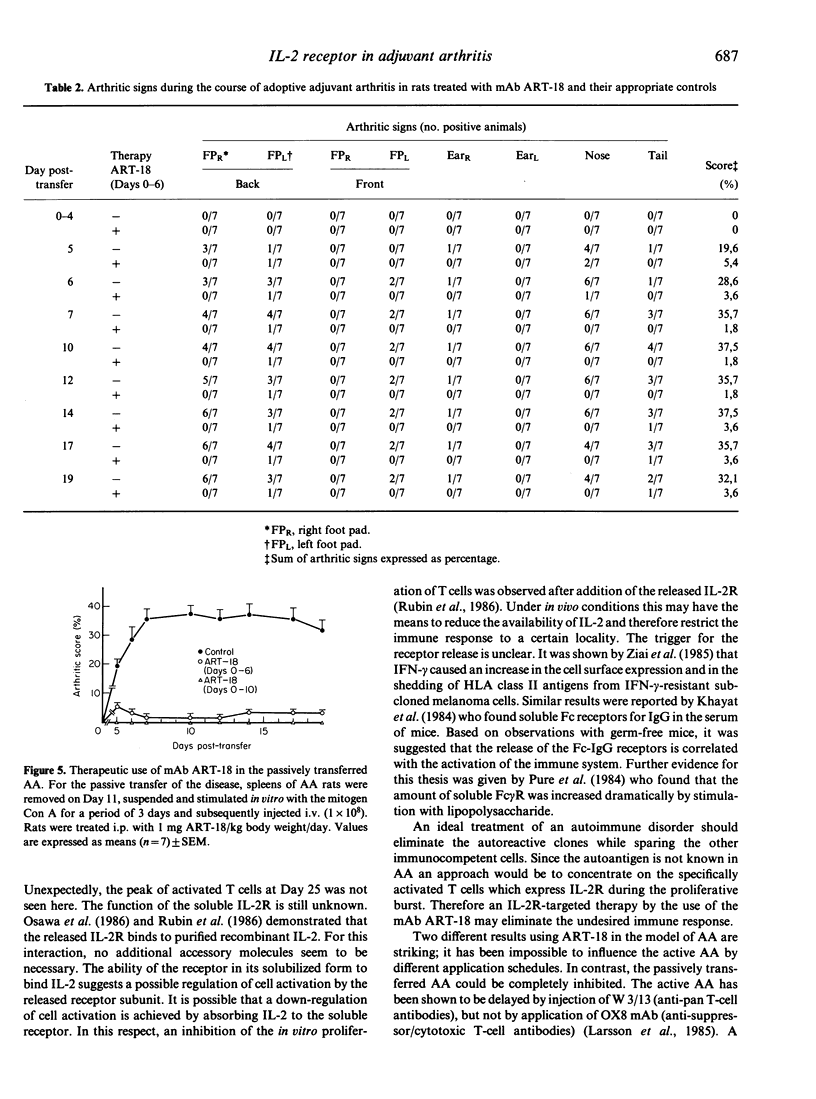
Recent evidence indicates that adjuvant arthritis (AA) of rats induced by complete Freund's adjuvant (CFA) is an autoimmune disease that is mediated by T cells. This report describes the distribution of activated IL-2 receptor (IL-2R)-bearing cells in spleen, popliteal lymph nodes (PLN) and blood in AA rats and in naive healthy rats using the monoclonal antibody (mAb) ART-18. It was found that in the primary lymph nodes (injected side) two peaks of elevated numbers of IL-2R-positive cells (Day 9/10 with a 40-fold increase; Day 25 with a 75-80-fold increase) occur. The PLN of the non-injected site also show an increase (30-fold) in the number of IL-2R-positive cells on Day 25. This investigation also included the monitoring of soluble IL-2R in the serum of AA rats in comparison to control sera of non-induced rats. The incidence of free IL-2R in the serum of AA rats does not completely correlate with the pattern of the distribution of receptor-bearing cells in PLN; elevated levels of IL-2R were observed at Day 9 and subsequently declined to below control levels. On Day 25, there was no correlation between IL-2R+ cells and soluble IL-2R. ART-18 was not active in suppressing the development of AA, in contrast to the complete inhibition of the passively transferred AA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan C. F., Leech S. H. The immunoregulatory nature of iron. I. Lymphocyte proliferation. Cell Immunol. 1983 Jan;75(1):71–79. doi: 10.1016/0008-8749(83)90306-4. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Larsson E. L., Grönvik K. O., Andersson J. Studies on T lymphocyte activation II. The target cells for concanavalin A-induced growth factors. Eur J Immunol. 1979 Aug;9(8):587–592. doi: 10.1002/eji.1830090803. [DOI] [PubMed] [Google Scholar]
- Diamantstein T., Osawa H. The interleukin-2 receptor, its physiology and a new approach to a selective immunosuppressive therapy by anti-interleukin-2 receptor monoclonal antibodies. Immunol Rev. 1986 Aug;92:5–27. doi: 10.1111/j.1600-065x.1986.tb01491.x. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Hancock W. W., Lord R. H., Colby A. J., Diamantstein T., Rickles F. R., Dijkstra C., Hogg N., Tilney N. L. Identification of IL 2R+ T cells and macrophages within rejecting rat cardiac allografts, and comparison of the effects of treatment with anti-IL 2R monoclonal antibody or cyclosporin. J Immunol. 1987 Jan 1;138(1):164–170. [PubMed] [Google Scholar]
- Herrmann F., Cannistra S. A., Levine H., Griffin J. D. Expression of interleukin 2 receptors and binding of interleukin 2 by gamma interferon-induced human leukemic and normal monocytic cells. J Exp Med. 1985 Sep 1;162(3):1111–1116. doi: 10.1084/jem.162.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J., Matitiau A., Cohen I. R. Arthritis induced in rats by cloned T lymphocytes responsive to mycobacteria but not to collagen type II. J Clin Invest. 1984 Jan;73(1):211–215. doi: 10.1172/JCI111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka Y., Chang Y. H. Adjuvant polyarthritis. VII. The role of type II collagen in pathogenesis. Arthritis Rheum. 1982 Nov;25(11):1325–1332. doi: 10.1002/art.1780251108. [DOI] [PubMed] [Google Scholar]
- Kayashima K., Koga T., Onoue K. Role of T lymphocytes in adjuvant arthritis. I. Evidence for the regulatory function of thymus-derived cells in the induction of the disease. J Immunol. 1976 Nov;117(5 PT2):1878–1882. [PubMed] [Google Scholar]
- Kelley V. E., Naor D., Tarcic N., Gaulton G. N., Strom T. B. Anti-interleukin 2 receptor antibody suppresses delayed-type hypersensitivity to foreign and syngeneic antigens. J Immunol. 1986 Oct 1;137(7):2122–2124. [PubMed] [Google Scholar]
- Kupiec-Weglinski J. W., Di Stefano R., Stunkel K. G., Grutzmann R., Theisen P., Araneda D., Tilney N. L., Diamantstein T. Anti-interleukin 2 receptor monoclonal antibody therapy in rat recipients of cardiac allografts: the role of antibody isotype. Transplant Proc. 1988 Feb;20(1 Suppl 1):272–275. [PubMed] [Google Scholar]
- Larsson P., Holmdahl R., Dencker L., Klareskog L. In vivo treatment with W3/13 (anti-pan T) but not with OX8 (anti-suppressor/cytotoxic T) monoclonal antibodies impedes the development of adjuvant arthritis in rats. Immunology. 1985 Nov;56(3):383–391. [PMC free article] [PubMed] [Google Scholar]
- Loken M. R., Herzenber L. A. Analysis of cell populations with a fluorescence-activated cell sorter. Ann N Y Acad Sci. 1975 Jun 30;254:163–171. doi: 10.1111/j.1749-6632.1975.tb29166.x. [DOI] [PubMed] [Google Scholar]
- Mouzaki A., Osawa H., Diamantstein T. An ELISA that detects cell-associated and released rat IL-2 receptors in a soluble form. J Immunol Methods. 1987 Jun 26;100(1-2):243–248. doi: 10.1016/0022-1759(87)90195-5. [DOI] [PubMed] [Google Scholar]
- Munker R., Stünkel K., Thiel E., Thierfelder S. Analysis with monoclonal antibodies of human lymphoid cells forming rosettes with rabbit red blood cells. Clin Exp Immunol. 1983 Mar;51(3):479–486. [PMC free article] [PubMed] [Google Scholar]
- Osawa H., Diamantstein T. Partial characterization of the putative rat interleukin 2 receptor. Eur J Immunol. 1984 Apr;14(4):374–376. doi: 10.1002/eji.1830140418. [DOI] [PubMed] [Google Scholar]
- Osawa H., Josimovic-Alasevic O., Diamantstein T. Enzyme-linked immunosorbent assay of mouse interleukin-2 receptors. J Immunol Methods. 1986 Aug 21;92(1):109–115. doi: 10.1016/0022-1759(86)90510-7. [DOI] [PubMed] [Google Scholar]
- PEARSON C. M. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956 Jan;91(1):95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- Pure E., Durie C. J., Summerill C. K., Unkeless J. C. Identification of soluble Fc receptors in mouse serum and the conditioned medium of stimulated B cells. J Exp Med. 1984 Dec 1;160(6):1836–1849. doi: 10.1084/jem.160.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. A., Jay G., Nelson D. L. The released interleukin 2 receptor binds interleukin 2 efficiently. J Immunol. 1986 Dec 15;137(12):3841–3844. [PubMed] [Google Scholar]
- Smith K. A., Gillis S., Baker P. E., McKenzie D., Ruscetti F. W. T-cell growth factor-mediated T-cell proliferation. Ann N Y Acad Sci. 1979;332:423–432. doi: 10.1111/j.1749-6632.1979.tb47136.x. [DOI] [PubMed] [Google Scholar]
- Volk H. D., Brocke S., Osawa H., Diamantstein T. Suppression of the local graft-vs.-host reaction in rats by treatment with a monoclonal antibody specific for the interleukin 2 receptor. Eur J Immunol. 1986 Oct;16(10):1309–1312. doi: 10.1002/eji.1830161020. [DOI] [PubMed] [Google Scholar]
- Ziai M. R., Imberti L., Tongson A., Ferrone S. Differential modulation by recombinant immune interferon of the expression and shedding of HLA antigens and melanoma associated antigens by a melanoma cell line resistant to the antiproliferative activity of immune interferon. Cancer Res. 1985 Nov;45(11 Pt 2):5877–5882. [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Nevo Z., Frenkel A., Klajman A., Cohen I. R. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5117–5120. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]


