Abstract
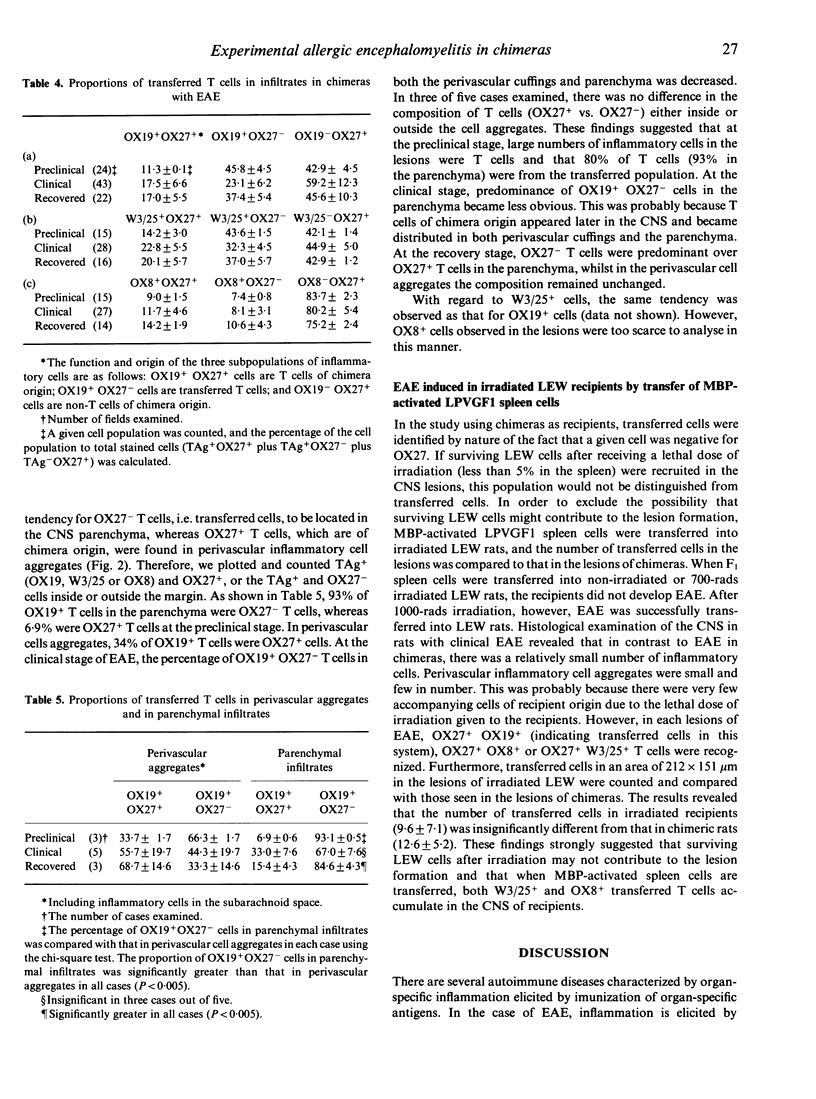
Experimental allergic encephalomyelitis (EAE) was induced by adoptive transfer of myelin basic protein (MBP)-activated LEW spleen cells into (LEW x PVG/c) F1----LEW chimeras. By double-immunofluorescent staining using OX27, which is specific for RT1c, and monoclonal antibodies (mAb) against various T-cell antigens (TAg), inflammatory cells in the lesions of the central nervous system (CNS) were categorized into MBP-activated and transferred LEW T cells (TAg+ OX27-), accompanying T cells (TAg+ OX27+) of chimera origin and non-T cells (TAg- OX27+). Examination of the lesions at various stages of EAE revealed that transferred OX19 (CD5)+ T cells accounted for 46% of the total number of inflammatory cells at the preclinical stage, became reduced to 23% at the clinical stage and recovered to a level between those of the preclinical and clinical stages at the recovery stage. In parenchymal infiltrates, 93% of the total T cells were transferred cells at the preclinical stage, whereas 66% were present in perivascular aggregates. At the clinical stage, the proportion of transferred T cells in the parenchyma was not different from that in the perivascular cuffs. At the recovery stage, the proportion of transferred T cells in the parenchyma was increased. Collectively, MBP-activated and transferred T cells first appeared in the CNS parenchyma followed by infiltration of T and non-T cells of recipient (chimera) origin. All these inflammatory cells formed the lesions of full-blown EAE. At the recovery stage, inflammatory cells decreased in number in all the compartments of the CNS. Transferred T cells formed the major proportion of parenchymal infiltrates at this stage. These findings strongly suggest that transferred T cells remain in the CNS parenchyma longer than cells of chimera origin and that antigen-activated T cells have well-expressed CNS-homing activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N. The localization of populations of lymphocytes defined by monoclonal antibodies in rat lymphoid tissues. Immunology. 1981 Apr;42(4):593–600. [PMC free article] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Caspi R. R., Roberge F. G., McAllister C. G., el-Saied M., Kuwabara T., Gery I., Hanna E., Nussenblatt R. B. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986 Feb 1;136(3):928–933. [PubMed] [Google Scholar]
- Dallman M. J., Thomas M. L., Green J. R. MRC OX-19: a monoclonal antibody that labels rat T lymphocytes and augments in vitro proliferative responses. Eur J Immunol. 1984 Mar;14(3):260–267. doi: 10.1002/eji.1830140311. [DOI] [PubMed] [Google Scholar]
- Endoh M., Tabira T., Kunishita T., Sakai K., Yamamura T., Taketomi T. DM-20, a proteolipid apoprotein, is an encephalitogen of acute and relapsing autoimmune encephalomyelitis in mice. J Immunol. 1986 Dec 15;137(12):3832–3835. [PubMed] [Google Scholar]
- Englert M. E., Ferguson K. M., Suarez C. R., Oronsky A. L., Kerwar S. S. Passive transfer of collagen arthritis: heterogeneity of anti-collagen IgG. Cell Immunol. 1986 Sep;101(2):373–379. doi: 10.1016/0008-8749(86)90150-4. [DOI] [PubMed] [Google Scholar]
- Izumo S., Linington C., Wekerle H., Meyermann R. Morphologic study on experimental allergic neuritis mediated by T cell line specific for bovine P2 protein in Lewis rats. Lab Invest. 1985 Aug;53(2):209–218. [PubMed] [Google Scholar]
- Linington C., Izumo S., Suzuki M., Uyemura K., Meyermann R., Wekerle H. A permanent rat T cell line that mediates experimental allergic neuritis in the Lewis rat in vivo. J Immunol. 1984 Oct;133(4):1946–1950. [PubMed] [Google Scholar]
- Mann R., Zakheim B., Clayman M., McCafferty E., Michaud L., Neilson E. G. Murine interstitial nephritis. IV. Long-term cultured L3T4+ T cell lines transfer delayed expression of disease as I-A-restricted inducers of the effector T cell repertoire. J Immunol. 1985 Jul;135(1):286–293. [PubMed] [Google Scholar]
- Maron R., Zerubavel R., Friedman A., Cohen I. R. T lymphocyte line specific for thyroglobulin produces or vaccinates against autoimmune thyroiditis in mice. J Immunol. 1983 Nov;131(5):2316–2322. [PubMed] [Google Scholar]
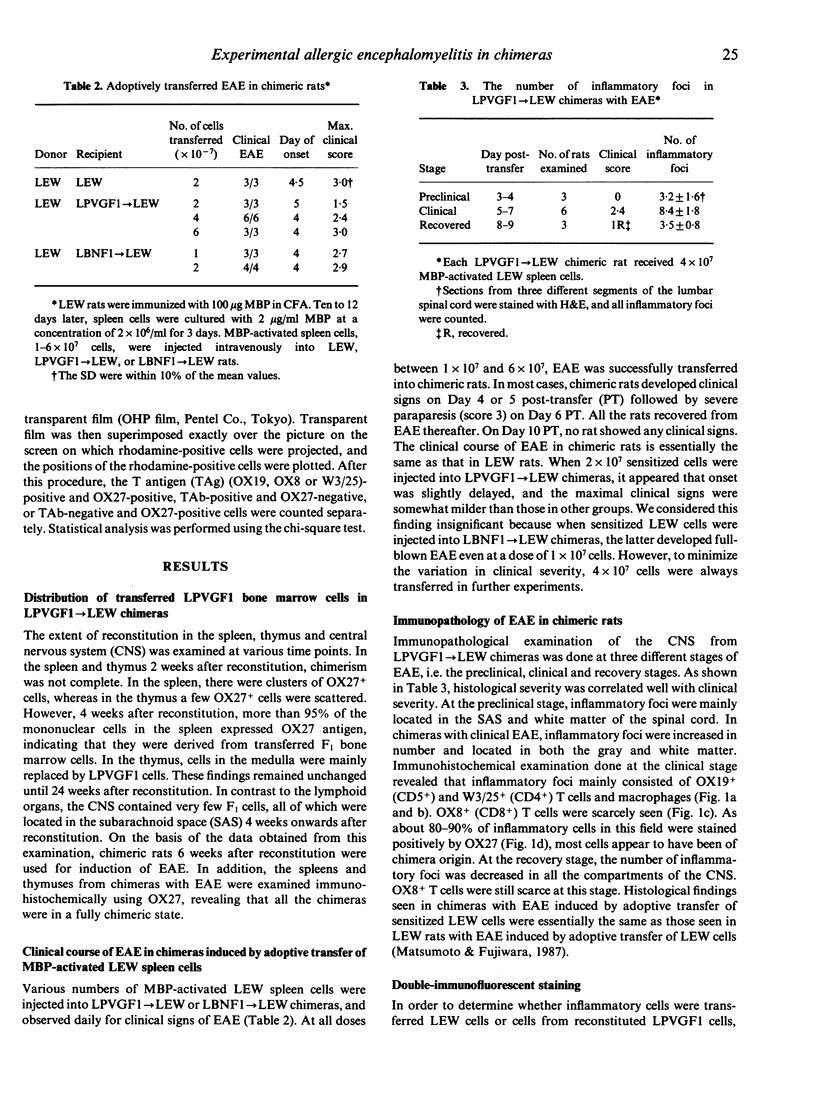
- Matsumoto Y., Hara N., Tanaka R., Fujiwara M. Immunohistochemical analysis of the rat central nervous system during experimental allergic encephalomyelitis, with special reference to Ia-positive cells with dendritic morphology. J Immunol. 1986 May 15;136(10):3668–3676. [PubMed] [Google Scholar]
- Naparstek Y., Ben-Nun A., Holoshitz J., Reshef T., Frenkel A., Rosenberg M., Cohen I. R. T lymphocyte lines producing or vaccinating against autoimmune encephalomyelitis (EAE). Functional activation induces peanut agglutinin receptors and accumulation in the brain and thymus of line cells. Eur J Immunol. 1983 May;13(5):418–423. doi: 10.1002/eji.1830130513. [DOI] [PubMed] [Google Scholar]
- Paterson D. J., Green J. R., Jefferies W. A., Puklavec M., Williams A. F. The MRC OX-44 antigen marks a functionally relevant subset among rat thymocytes. J Exp Med. 1987 Jan 1;165(1):1–13. doi: 10.1084/jem.165.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert J. R., Driscoll B. F., Kies M. W., Alvord E. C., Jr Adoptive transfer of experimental allergic encephalomyelitis: incubation of rat spleen cells with specific antigen. J Immunol. 1979 Feb;122(2):494–496. [PubMed] [Google Scholar]
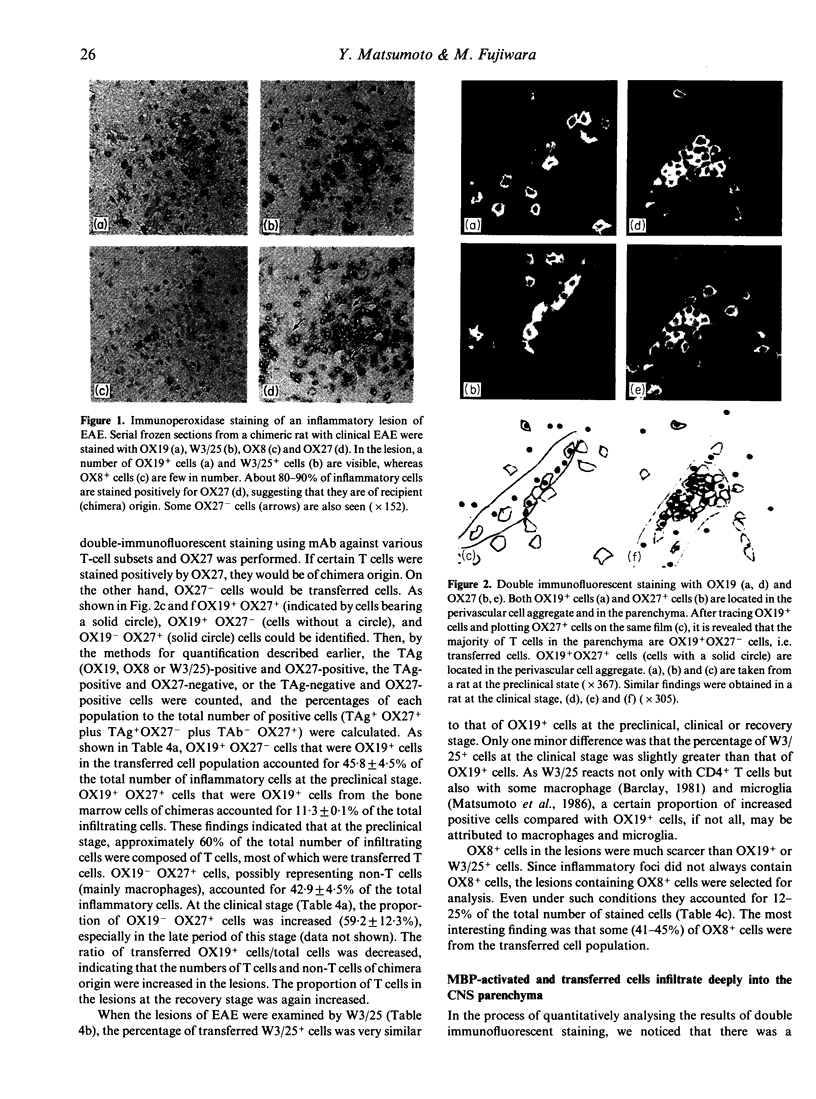
- Robinson A. P., White T. M., Mason D. W. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986 Feb;57(2):239–247. [PMC free article] [PubMed] [Google Scholar]
- Romball C. G., Weigle W. O. Transfer of experimental autoimmune thyroiditis with T cell clones. J Immunol. 1987 Feb 15;138(4):1092–1098. [PubMed] [Google Scholar]
- Rozenszajn L. A., Muellenberg-Coulombre C., Gery I., el-Saied M., Kuwabara T., Mochizuki M., Lando Z., Nussenblatt R. B. Induction of experimental autoimmune uveoretinitis by T-cell lines. Immunology. 1986 Apr;57(4):559–565. [PMC free article] [PubMed] [Google Scholar]
- Satoh J., Sakai K., Endoh M., Koike F., Kunishita T., Namikawa T., Yamamura T., Tabira T. Experimental allergic encephalomyelitis mediated by murine encephalitogenic T cell lines specific for myelin proteolipid apoprotein. J Immunol. 1987 Jan 1;138(1):179–184. [PubMed] [Google Scholar]
- Singer D. E., Moore M. J., Williams R. M. EAE in rat bone marrow chimeras: analysis of the cellular mechanism of BN resistance. J Immunol. 1981 Apr;126(4):1553–1557. [PubMed] [Google Scholar]
- Smith S. B., Waksman B. H. Passive transfer and labelling studies on the cell infiltrate in experimental allergic encephalomyelitis. J Pathol. 1969 Nov;99(3):237–244. doi: 10.1002/path.1710990307. [DOI] [PubMed] [Google Scholar]
- Tung K. S., Yule T. D., Mahi-Brown C. A., Listrom M. B. Distribution of histopathology and Ia positive cells in actively induced and passively transferred experimental autoimmune orchitis. J Immunol. 1987 Feb 1;138(3):752–759. [PubMed] [Google Scholar]
- Vandenbark A. A., Gill T., Offner H. A myelin basic protein-specific T lymphocyte line that mediates experimental autoimmune encephalomyelitis. J Immunol. 1985 Jul;135(1):223–228. [PubMed] [Google Scholar]
- Vass K., Lassmann H., Wekerle H., Wisniewski H. M. The distribution of Ia antigen in the lesions of rat acute experimental allergic encephalomyelitis. Acta Neuropathol. 1986;70(2):149–160. doi: 10.1007/BF00691433. [DOI] [PubMed] [Google Scholar]
- White R. A., Mason D. W., Williams A. F., Galfre G., Milstein C. T-lymphocyte heterogeneity in the rat: separation of functional subpopulations using a monoclonal antibody. J Exp Med. 1978 Sep 1;148(3):664–673. doi: 10.1084/jem.148.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Yamamura T., Namikawa T., Endoh M., Kunishita T., Tabira T. Experimental allergic encephalomyelitis induced by proteolipid apoprotein in Lewis rats. J Neuroimmunol. 1986 Aug;12(2):143–153. doi: 10.1016/0165-5728(86)90027-5. [DOI] [PubMed] [Google Scholar]
- Zakheim B., McCafferty E., Phillips S. M., Clayman M., Neilson E. G. Murine interstitial nephritis. II. The adoptive transfer of disease with immune T lymphocytes produces a phenotypically complex interstitial lesion. J Immunol. 1984 Jul;133(1):234–239. [PubMed] [Google Scholar]
- Zamvil S., Nelson P., Trotter J., Mitchell D., Knobler R., Fritz R., Steinman L. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. 1985 Sep 26-Oct 2Nature. 317(6035):355–358. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Nevo Z., Frenkel A., Klajman A., Cohen I. R. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5117–5120. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]