Abstract
Decay-accelerating factor (DAF), a membrane protein that regulates the complement system, was purified to homogeneity from lymphoblastoid (Raji) cells (DAF-R). It exhibited almost the same molecular weight as DAF from stroma of erythrocytes (DAF-S). Purified DAF-R, which could be reincorporated into cell membranes, accelerated the decay of the C3 convertases, in both the classical (C4b2a) and the alternative (C3bBb) pathways. This activity was completely inhibited by a monoclonal anti-DAF antibody, 1C6. From these results, DAF-R and DAF-S can not be distinguished; however, the decay-accelerating activity of DAF-R was much higher than that of DAF-S. 1C6 enhanced the binding of C3 to Raji cells by incubating with six purified components of the alternative pathway, whereas it did not induce the killing of Raji cells after a short incubation period. When antibodies against Raji cells were added to the above system, the blocking of DAF activity by 1C6 resulted in efficient killing of Raji cells by autologous complement. From these results, it is clear that DAF on nucleated cells plays an important role in protecting these cells from the damage caused by autologous complement.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Kinoshita T., Jaffe E. A., Nussenzweig V. Decay-accelerating factor is present on cultured human umbilical vein endothelial cells. J Exp Med. 1986 Jan 1;163(1):221–226. doi: 10.1084/jem.163.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boenisch T., Alper C. A. Isolation and properties of a glycine-rich beta-glycoprotein of human serum. Biochim Biophys Acta. 1970 Dec 22;221(3):529–535. doi: 10.1016/0005-2795(70)90224-2. [DOI] [PubMed] [Google Scholar]
- Bolotin C., Morris S., Tack B., Prahl J. Purification and structural analysis of the fourth component of human complement. Biochemistry. 1977 May 3;16(9):2008–2015. doi: 10.1021/bi00628a039. [DOI] [PubMed] [Google Scholar]
- Budzko D. B., Lachmann P. J., McConnell I. Activation of the alternative complement pathway by lymphoblastoid cell lines derived from patients with Burkitt's lymphoma and infectious mononucleosis. Cell Immunol. 1976 Mar 1;22(1):98–109. doi: 10.1016/0008-8749(76)90011-3. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Low M. G., Nussenzweig V. Release of decay-accelerating factor (DAF) from the cell membrane by phosphatidylinositol-specific phospholipase C (PIPLC). Selective modification of a complement regulatory protein. J Exp Med. 1986 May 1;163(5):1150–1161. doi: 10.1084/jem.163.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Purification of C3b inactivator and demonstration of its two polypeptide chain structure. J Immunol. 1977 Oct;119(4):1248–1252. [PubMed] [Google Scholar]
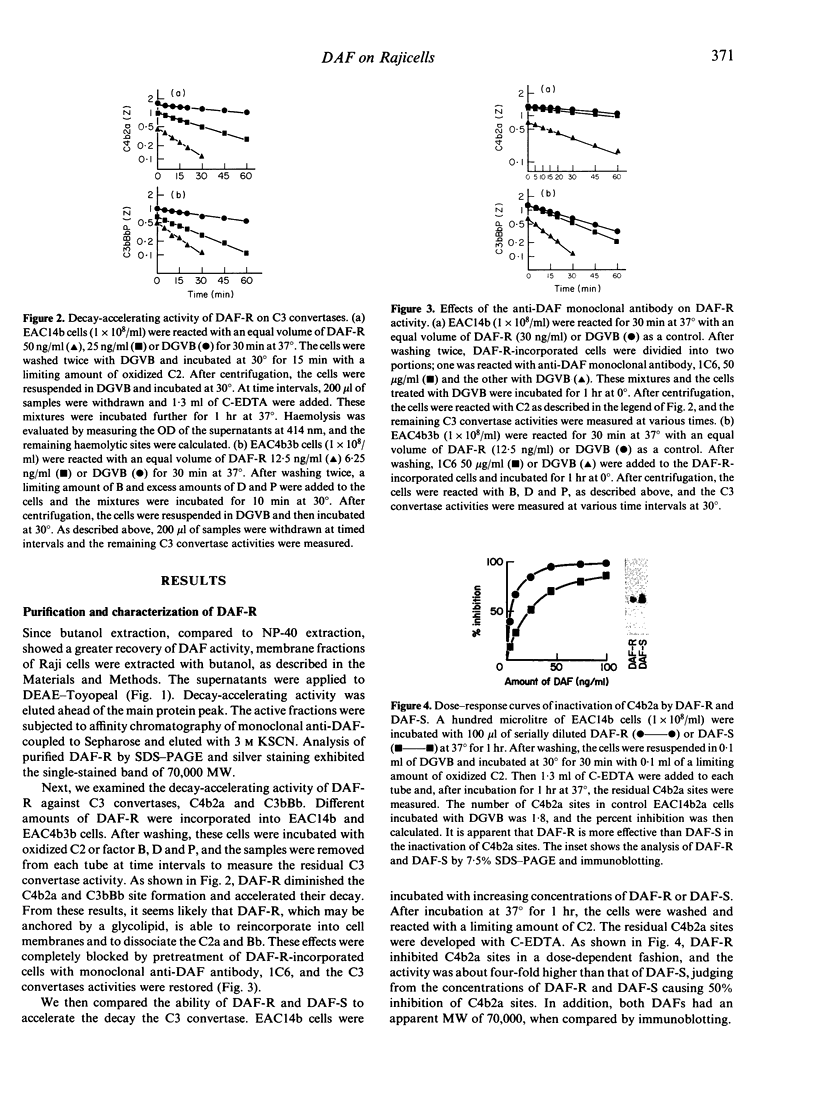
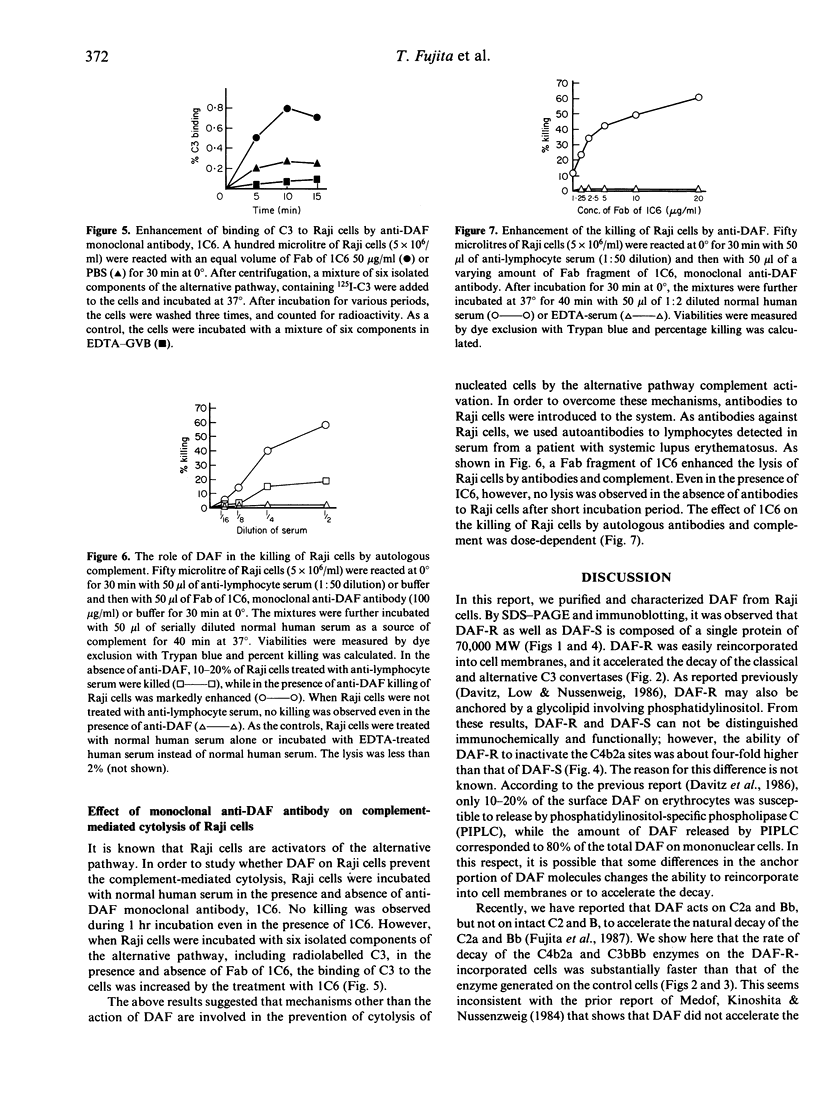
- Fujita T., Inoue T., Ogawa K., Iida K., Tamura N. The mechanism of action of decay-accelerating factor (DAF). DAF inhibits the assembly of C3 convertases by dissociating C2a and Bb. J Exp Med. 1987 Nov 1;166(5):1221–1228. doi: 10.1084/jem.166.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Takata Y., Tamura N. Solubilization of immune precipitates by six isolated alternative pathway proteins. J Exp Med. 1981 Dec 1;154(6):1743–1751. doi: 10.1084/jem.154.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Tamura N. Interaction of C4-binding protein with cell-bound C4b. A quantitative analysis of binding and the role of C4-binding protein in proteolysis of cell-bound C4b. J Exp Med. 1983 Apr 1;157(4):1239–1251. doi: 10.1084/jem.157.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E. M. Inhibition of complement by a substance isolated from human erythrocytes. II. Studies on the site and mechanism of action. Immunochemistry. 1969 May;6(3):405–419. doi: 10.1016/0019-2791(69)90297-3. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Silber R., Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985 Jul 1;162(1):75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lesavre P. H., Hugli T. E., Esser A. F., Müller-Eberhard H. J. The alternative pathway C3/C5 convertase: chemical basis of factor B activation. J Immunol. 1979 Aug;123(2):529–534. [PubMed] [Google Scholar]
- Medicus R. G., Esser A. F., Fernandez H. N., Müller-Eberhard H. J. Native and activated properdin: interconvertibility and identity of amino- and carboxy-terminal sequences. J Immunol. 1980 Feb;124(2):602–606. [PubMed] [Google Scholar]
- Morgan B. P., Dankert J. R., Esser A. F. Recovery of human neutrophils from complement attack: removal of the membrane attack complex by endocytosis and exocytosis. J Immunol. 1987 Jan 1;138(1):246–253. [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosen C. E., Spicer D. B., Austen K. F. Surface membrane expression by human blood leukocytes and platelets of decay-accelerating factor, a regulatory protein of the complement system. Blood. 1985 May;65(5):1237–1244. [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosenfeld S. I., Austen K. F. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5066–5070. doi: 10.1073/pnas.80.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5430–5434. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P., Androlewicz M. J., Brodsky F. M., Holmes N. J., Ways J. P. Monoclonal antibodies: purification, fragmentation and application to structural and functional studies of class I MHC antigens. J Immunol Methods. 1982 Sep 17;53(2):133–173. doi: 10.1016/0022-1759(82)90137-5. [DOI] [PubMed] [Google Scholar]
- Polley M. J., Müller-Eberhard H. J. Enharncement of the hemolytic activity of the second component of human complement by oxidation. J Exp Med. 1967 Dec 1;126(6):1013–1025. doi: 10.1084/jem.126.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönermark S., Rauterberg E. W., Shin M. L., Löke S., Roelcke D., Hänsch G. M. Homologous species restriction in lysis of human erythrocytes: a membrane-derived protein with C8-binding capacity functions as an inhibitor. J Immunol. 1986 Mar 1;136(5):1772–1776. [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalman L. S., Wood L. M., Müller-Eberhard H. J. Isolation of a human erythrocyte membrane protein capable of inhibiting expression of homologous complement transmembrane channels. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6975–6979. doi: 10.1073/pnas.83.18.6975. [DOI] [PMC free article] [PubMed] [Google Scholar]