Abstract
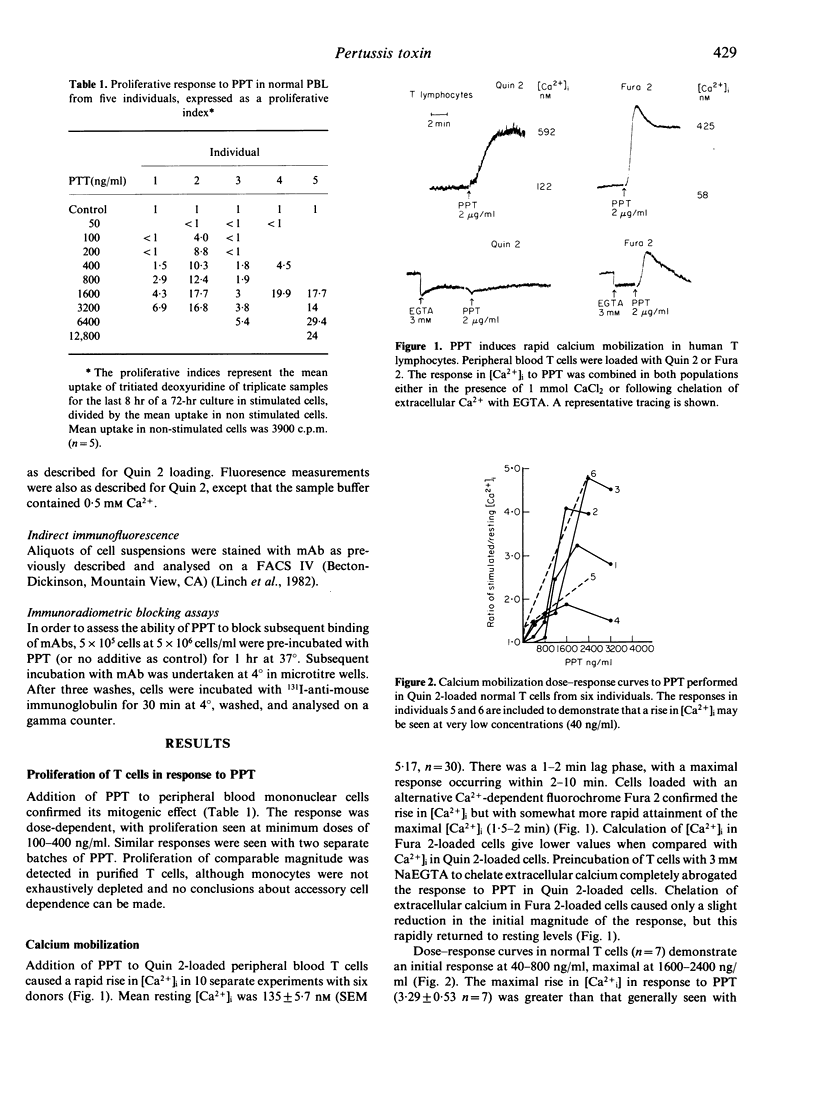
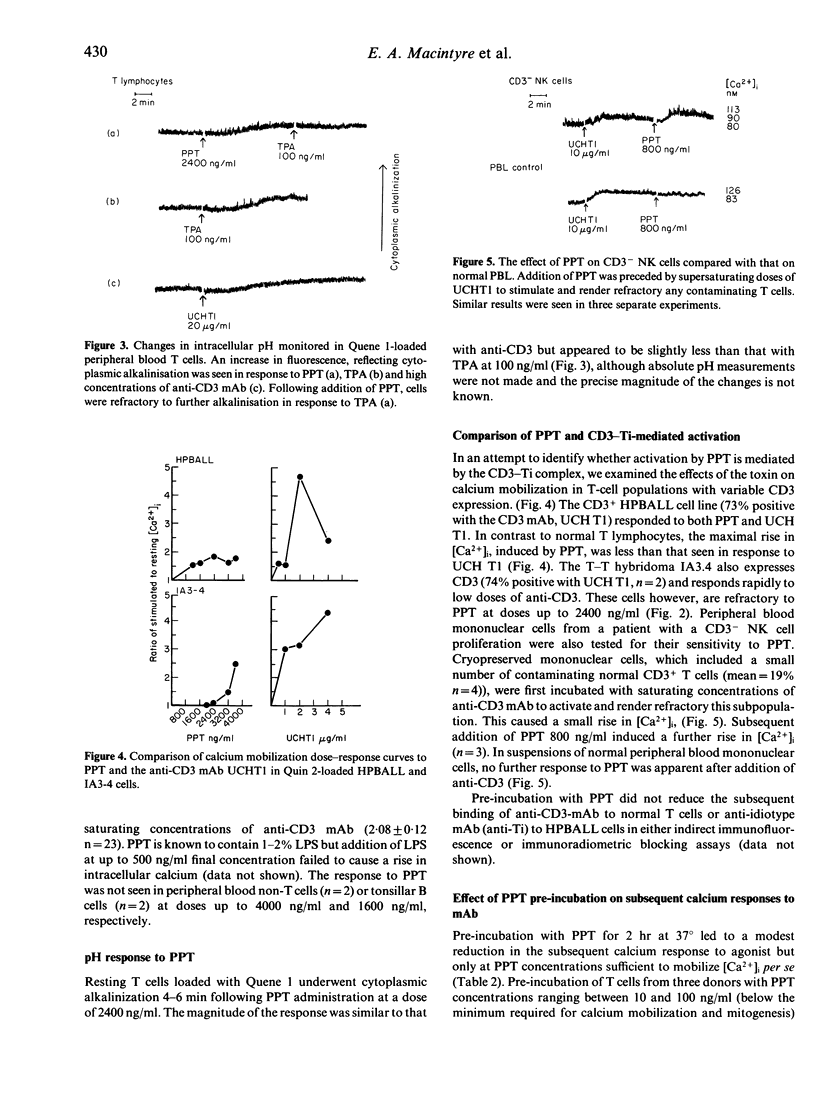
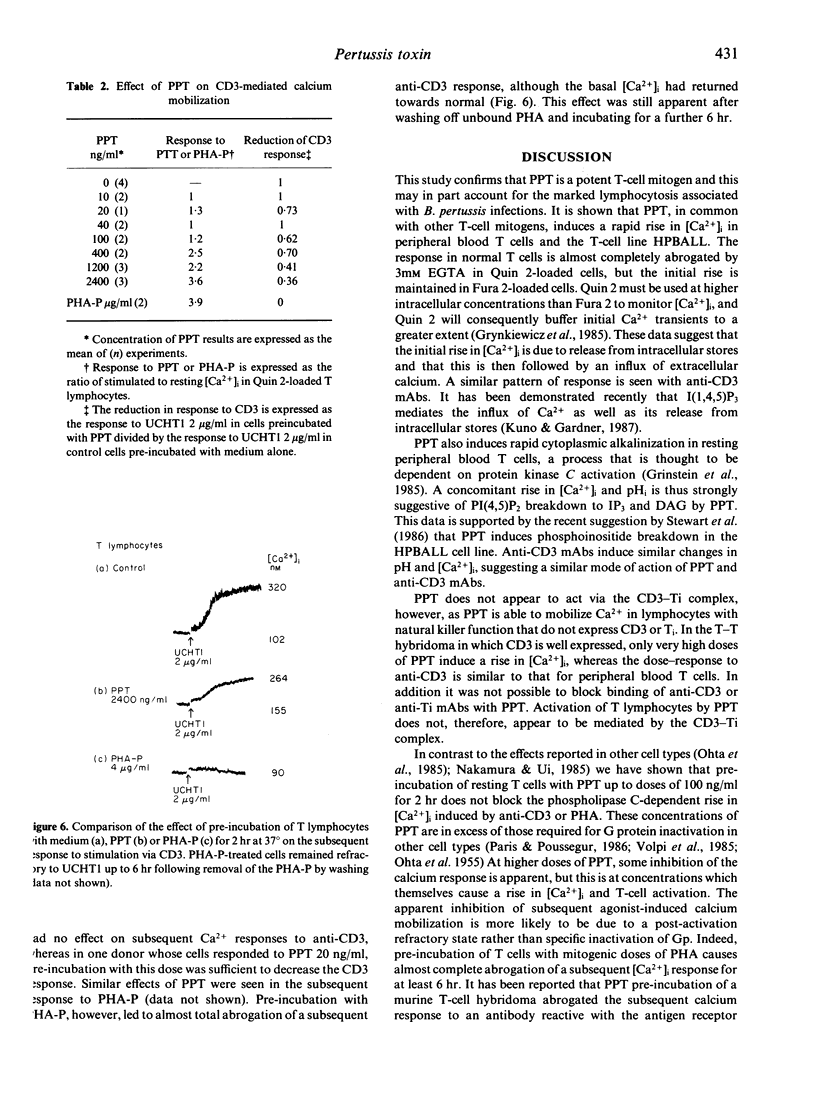
Purified pertussis toxin (PPT) is a potent mitogen for human T lymphocytes and is shown to cause rapid calcium mobilization in resting T cells, a T-cell line and CD3- lymphocytes with natural killer (NK) activity. In resting T cells the PPT activation is associated with cytoplasmic alkalinization. A similar rise in intracellular free calcium ([Ca2+]i) and cytoplasmic alkalinization is observed with activation through the antigen receptor complex. The effect of PPT is unlikely to be mediated through this pathway, however, as it can mobilize calcium in lymphocytes that do not express the CD3-Ti complex. In contrast to several other cell types, re-incubation of resting human T cells with PPT, up to a dose of 100 ng/ml for 2 hr does not block subsequent agonist-induced calcium mobilization dependent on G protein-mediated phospholipase C activation. Mitogenic doses of PPT cause a modest reduction in subsequent agonist responses, but this is likely to be due to a post-activation refractory state rather than G protein inactivation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando I., Hariri G., Wallace D., Beverley P. Tumor promoter phorbol esters induce unresponsiveness to antigen and expression of interleukin 2 receptor on T cells. Eur J Immunol. 1985 Feb;15(2):196–199. doi: 10.1002/eji.1830150217. [DOI] [PubMed] [Google Scholar]
- Barrowman M. M., Cockcroft S., Gomperts B. D. Differential control of azurophilic and specific granule exocytosis in Sendai-virus-permeabilized rabbit neutrophils. J Physiol. 1987 Feb;383:115–124. doi: 10.1113/jphysiol.1987.sp016399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Boylston A. W., Goldin R. D., Moore C. S. A human T cell tumor which expresses the putative T cell antigen receptor. Eur J Immunol. 1984 Mar;14(3):273–275. doi: 10.1002/eji.1830140313. [DOI] [PubMed] [Google Scholar]
- Chambard J. C., Paris S., L'Allemain G., Pouysségur J. Two growth factor signalling pathways in fibroblasts distinguished by pertussis toxin. Nature. 1987 Apr 23;326(6115):800–803. doi: 10.1038/326800a0. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Cohen S., Goetz J. D., Rothstein A., Gelfand E. W. Characterization of the activation of Na+/H+ exchange in lymphocytes by phorbol esters: change in cytoplasmic pH dependence of the antiport. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1429–1433. doi: 10.1073/pnas.82.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987 Mar 19;326(6110):301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Linch D. C., Beverley P. C., Levinsky R. J., Rodeck C. H. Phenotypic analysis of fetal blood leucocytes: potential for prenatal diagnosis of immunodeficiency disorders. Prenat Diagn. 1982 Jul;2(3):211–218. doi: 10.1002/pd.1970020310. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hodgdon J. C., Hussey R. E., Protentis J. P., Schlossman S. F., Reinherz E. L. Antigen-like effects of monoclonal antibodies directed at receptors on human T cell clones. J Exp Med. 1983 Sep 1;158(3):988–993. doi: 10.1084/jem.158.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowada J., Janossy G., Greaves M. F., Tsubota T., Srivastava B. I., Morikawa S., Tatsumi E. Expression of an antigen associated with acute lymphoblastic leukemia in human leukemia-lymphoma cell lines. J Natl Cancer Inst. 1978 Jun;60(6):1269–1277. doi: 10.1093/jnci/60.6.1269. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tsien R. Y., van der Saag P. T., de Laat S. W. Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature. 1983 Aug 18;304(5927):645–648. doi: 10.1038/304645a0. [DOI] [PubMed] [Google Scholar]
- Morse J. H., Kong A. S., Lindenbaum J., Morse S. I. The mitogenic effect of the lymphocytosis promoting factor from Bordetella pertussis on human lymphocytes. J Clin Invest. 1977 Sep;60(3):683–692. doi: 10.1172/JCI108820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985 Mar 25;260(6):3584–3593. [PubMed] [Google Scholar]
- O'Flynn K., Knott L. J., Russul-Saib M., Abdul-Gaffar R., Morgan G., Beverley P. C., Linch D. C. CD2 and CD3 antigens mobilize Ca2+ independently. Eur J Immunol. 1986 May;16(5):580–584. doi: 10.1002/eji.1830160521. [DOI] [PubMed] [Google Scholar]
- O'Flynn K., Linch D. C., Tatham P. E. The effect of mitogenic lectins and monoclonal antibodies on intracellular free calcium concentration in human T-lymphocytes. Biochem J. 1984 Apr 15;219(2):661–666. doi: 10.1042/bj2190661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H., Okajima F., Ui M. Inhibition by islet-activating protein of a chemotactic peptide-induced early breakdown of inositol phospholipids and Ca2+ mobilization in guinea pig neutrophils. J Biol Chem. 1985 Dec 15;260(29):15771–15780. [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Pertussis toxin inhibits thrombin-induced activation of phosphoinositide hydrolysis and Na+/H+ exchange in hamster fibroblasts. EMBO J. 1986 Jan;5(1):55–60. doi: 10.1002/j.1460-2075.1986.tb04177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truneh A., Albert F., Golstein P., Schmitt-Verhulst A. M. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985 Jan 24;313(6000):318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- Volpi M., Naccache P. H., Molski T. F., Shefcyk J., Huang C. K., Marsh M. L., Munoz J., Becker E. L., Sha'afi R. I. Pertussis toxin inhibits fMet-Leu-Phe- but not phorbol ester-stimulated changes in rabbit neutrophils: role of G proteins in excitation response coupling. Proc Natl Acad Sci U S A. 1985 May;82(9):2708–2712. doi: 10.1073/pnas.82.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]


