Full text
Breast cancer is the commonest malignancy among women in most European countries. Given current patterns of occurrence by age, about one in 12 women will develop the disease before the age of 75 years (lifetime risk around 8%), and it typically accounts for 20% or more of all cancers in women [1]. Breast cancer is thus a major public health problem, but with the exception of oral contraceptives and hormonal replacement therapy [2,3], both of which also have clear benefits, the known major risk factors are not amenable to primary prevention. Mass screening of women over age 50 years by mammography has been shown to reduce mortality [4]; national mammographic screening programmes were introduced for women aged 50-64 years in the UK from 1988 [5] and for women aged 50-70 years in The Netherlands from 1990 [6], and a regional scheme in Denmark was started in 1991 [7]. Opportunistic screening is also widespread in many European countries. The incidence of breast cancer has been increasing in many countries [1,8,9,10,11], but the available treatments have improved [12], survival has improved [13,14] and mortality has begun to decline [15].
Until recently, international comparisons of regional or national survival estimates within Europe were bedevilled by the lack of comparability of data and methods. Within the past 5 years, however, evidence of international differences in survival from breast cancer [16,17,18] (and many other cancers) in Europe has begun to emerge from the EUROCARE (European Cancer Registry-based study of survival and care of cancer patients) study. (The author has been a member of the EUROCARE Project Management Group since 1989, and is a co-author of various publications emanating from the project.) In this article, the evidence for these differences in breast cancer survival among women in Europe is reviewed, and some of the possible explanations are considered, with particular reference to the patterns of survival in the UK and the extent to which they differ from those of other countries in Europe.
The EUROCARE study now covers 17 European countries and is the largest international study of cancer survival. It includes 3.5 million cancer patients who were diagnosed between 1978 and 1989, and who have been followed up for at least 5 years, until the end of 1994 [19]. Data supplied in a standard format by 45 cancer registries covering a total population of about 100 million have been subjected to central quality control and analyzed using standard methods [20]. Relative survival up to 10 years after diagnosis by age, sex and country has been estimated for each of 45 cancers in adults aged 15-99 years at diagnosis.
For women diagnosed with breast cancer during the period 1978-1985, 5-year survival in 12 countries up to the end of 1990 ranged from 76% in Switzerland to 44% in Poland (Fig. 1a). In Switzerland and Finland, survival was significantly higher than the weighted European average for participating countries, and significantly lower in England, Scotland, Estonia and Poland [16].
Figure 1.
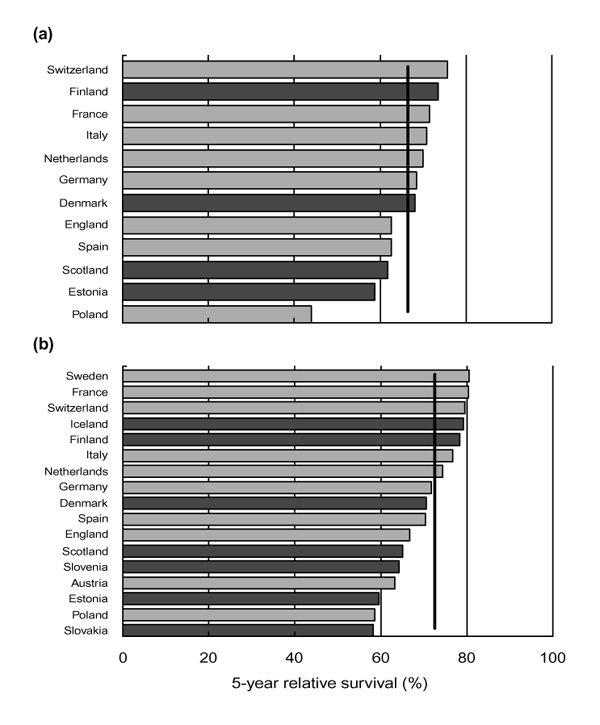
Age-standardized 5-year relative survival (%) from breast cancer in Europe. (a) Women diagnosed 1978-1985, followed up to 1990 [16]. (b) Women diagnosed 1985-1989, followed up to 1994 [18], National data sets are represented by darker bars and regional or subnational data sets by lighter bars. The vertical line is the weighted European average relative survival at 5 years.
Detailed analyses of more than 119000 women with breast cancer showed that survival improved in all 12 countries; the hazard of death fell by about 2.5% a year for each year of diagnosis between 1978 and 1985 [13]. Women aged 40-49 years at diagnosis had the best prognosis in all countries, whereas those under 30 years old had lower survival than those aged 30-39 years. The hazard of death within the first 6months after diagnosis was more steeply age-dependent than after the first 6 months, but gains in survival over time were most marked among age groups for which survival was initially the lowest (under 40 and over 80 years old). Within the first 6 months after diagnosis, the risk of death in each age group (particularly the youngest and oldest women) was 1.5-3 times higher in England and Scotland than the average for other countries. This comparison is statistically robust; it was based on more than 8700 deaths within the first 6months in the UK, and more than 3800 in the other countries. The relative risk of death for women with breast cancer in the UK fell at more than 6months after diagnosis, but was still higher in all age groups than the average for other countries. These patterns suggest that later diagnosis or more advanced stage is likely to explain some of the survival deficit in the UK, but that less effective treatment might also play a role.
More recent analyses [18] included 145000 women diagnosed in 17 countries during the period 1985-1989 who were followed up for at least 5 years to the end of 1994 (Fig. 1b). The average 5-year survival rate was 73%, but it ranged from 58-60% in Eastern Europe (Poland, Estonia and Slovakia) to 77-81% in Northern and Western Europe (Sweden, Finland, Iceland, Switzerland, France and Italy). Five-year survival in England, Scotland and Denmark (67-71%) was lower than the European average. Patterns of survival by age were again similar in each country, and in most countries survival improved over time.
The survival rates reported from EUROCARE include all cancer patients, take account of all causes of death and are age standardized. In this, they differ from the survival rates in randomized clinical trials and hospital studies in several key respects.
First, although randomized trials measure the best achievable survival, population studies measure the average survival actually achieved. Clinical trials show what survival rates are possible under ideal conditions, with the latest diagnostic techniques and treatment regimens for selected cancer patients in the care of experienced oncologists, often in specialized hospitals. Trials rarely include more than a small fraction of patients with a given cancer, and incorporation of the results into routine clinical practice can be slow, either because of delay in acceptance or lack of equipment or resources [21]. Trials often exclude elderly patients [22], even though opinion varies widely on how to treat breast cancer, both for elderly women [23] and for those under 50 years old [24], and older patients are less likely to be referred for specialist advice [25]. For all these reasons, cancer survival among patients recruited in clinical trials is inevitably higher than the average for all cancer patients. Survival estimates from trials are essential for assessing new treatments, but long-term data series from population-based cancer registries are equally indispensable to assess trends in the survival of all cancer patients [26]. Observational studies are the public health counterpart of clinical trials. When comparable data from many countries are compiled and analyzed to common standards, it becomes possible to make international comparisons of cancer survival rates and of trends in survival over time. Randomized trials and nonrandomized hospital studies of cancer survival do not address these questions.
Second, although cancer patients have higher death rates than the general population, they do not all die of cancer. This 'background' mortality among cancer patients varies with age and sex, but also between countries and over time. Relative survival rates take account of background mortality. A relative survival rate (say, 67%) is the ratio of the observed survival of a group of cancer patients (say, 60%) to the survival that they would have experienced had they only died at the same rate as the general population from which they came, matched for age, sex and geographic region or country (say, 90%). Thus, a relative survival rate of 100% would imply that the mortality of the cancer patients was the same as that of the general population, not that no-one died. It can be interpreted as the survival of cancer patients after adjustment for other causes of death [27]. Background mortality varies by as much as twofold across Europe, so relative survival rates are required for appropriate comparison of cancer survival between countries. The age distribution of cancer patients also varies between countries, however, and because relative survival varies with age, international cancer survival comparisons are adjusted to a common age standard. Trends in relative survival reflect both improvements in treatment and the extent to which they have become available to all cancer patients.
Survival from breast cancer (and many other cancers) varies with socioeconomic status and region of residence, both within the UK [14,28,29] and elsewhere in Europe [17]. Geographic and socioeconomic differences in investigation and treatment have been reported for patients with cancers of the breast [30,31,32] and variations in investigation or departure from treatment guidelines have caused inequity in survival [24,33,34].
The sensible question is no longer whether international, regional and socioeconomic differences in cancer survival exist, but why. The explanations are certain to be multiple, but the observed differences are not simply due to artefact [13,19,35], chance [36,37], or (solely) to the extent of disease at the time of diagnosis [38,39]. Delay in diagnosis is likely to reduce survival [40], however, and the risk of death among women with breast cancer in the UK in the first 6 months after diagnosis has been higher than that in other European countries [13]. International or regional differences in survival could be at least partly attributable to cultural differences that influence the stage at which disease is diagnosed, as well as to the different ways in which national health care systems are organized.
The clinical stage of disease at diagnosis is a key prognostic factor. Ideally, survival estimates would be adjusted for case-mix when making comparisons. International comparisons of cancer survival trends after adjustment for clinical stage are complex for three main reasons. First, an explicit and reliable statement of the clinical stage may not be available in the medical record [30]. Second, not all cancer registries have systematically recorded clinical stage at diagnosis over long periods. Third, the clinical stage recorded at diagnosis depends on the extent of investigation, which itself varies between centres and countries, and the diagnostic basis for the recorded clinical stage must therefore be taken into account [35,41]. Again, this information is not routinely available, but random samples of cancer patients are now being studied within the EUROCARE project to enable appropriate comparison of stage-adjusted survival rates between populations. Clearly, to the extent that lower survival in a given population can be shown to depend on the disease being diagnosed at a later stage, as for example in women over age 65 years living in deprived areas [39], it becomes possible to devise a suitable strategy for earlier diagnosis.
Treatment protocols for breast cancer vary widely, and adherence to guidelines in the UK appears poor [23,32,42]. The UK boasts fewer oncologists per head of population than most comparable European countries [43], and there is some evidence that breast cancer survival depends on access to a specialist [44]. A recent audit in the UK showed that 28% of cancer patients waited longer to receive radiotherapy than the maximum acceptable delay set out in professional guidelines; this was attributed to lack of equipment and staff [45]. The government acknowledged inequitable access to optimal cancer treatment in the National Health Service in 1995 [46]. Cancer survival in Denmark is also lower than that in the other Nordic countries, at least in part because Danish cancer patients appear to be diagnosed at a later stage [47].
It has been suggested that international differences in cancer survival, especially those reported from the EURO-CARE study between the UK and other European countries, are more likely to be due to demographic differences and to the certification of cancer patients outside the UK as having died of old age, and not cancer [48].
These suggestions are not tenable. The EUROCARE data include essentially all cancer patients resident in the participating regions or countries, and they are not subject to the selection bias of most hospital case series. Differences in background mortality by age, sex, calendar period and region or country are taken into account in the EUROCARE study, and because the relative survival rates are age-standardized, they also account for differences in the age distribution of cancer patients between countries. Complaints about inaccurate certification of the cause of death for cancer patients in other countries are not only misplaced (because there is ample evidence of inaccurate certification in the UK [49,50,51,52]), they are actually beside the point. Inaccurate certification of death could certainly influence recorded cancer mortality rates, but relative survival rates simply reflect the proportion of cancer patients who are still alive at a given time since diagnosis, compared with the proportion that would be expected among a corresponding group of the general population in the region or country in which they live. They are not affected by the certified cause of death. Uncertainties about the quality of death certification and the reliability with which a given death can be attributed as due to a previously diagnosed cancer actually underpin the rationale for relative survival rates as the most reliable measure for comparisons of survival between countries and over time [53,54].
In fact, the evidence suggests that many of the observed differences in breast cancer survival between countries, regions and population subgroups are systematic, and can be largely attributed to differences in access to health services, including delay in presentation and diagnosis, and to the overall quality of care [17,18,20]. Thus, for example, elimination of the socioeconomic gradient in breast cancer survival in England and Wales would eliminate each year more than 500 of the breast cancer deaths that now occur within 5years of diagnosis. It would also halve the deficit in breast cancer survival between England and Wales and the average for Europe [14]. It is hard to imagine any set of biases in data collection or analysis that could account for survival being lower in England and in Scotland than in most comparable European countries for breast cancer in women (and for many other cancers in both sexes), but as high as or higher than the European average survival for breast cancer in men (and for Hodgkin's disease and cancers of the larynx and testis). It would also be remarkable for any set of biases to result in such similar dependence of survival with age at diagnosis in each of 17 countries.
In July 1999, the UK government acknowledged that the UK lags behind other European countries in cancer survival [55]: an uncomfortable but salutary recognition of the need for improvement. Such improvement should be part of a broad strategy to control cancer. It will require more rapid referral from primary care to hospital, more rigorous adherence to consensus guidelines, and, perhaps above all, the availability of resources for enough specialists to deliver the best available treatment to all breast cancer patients.
Improvements in breast cancer survival for the population as a whole will also require that diagnosis is made at an earlier stage of the disease, and this in turn implies better public awareness of the need for early diagnosis and its impact on the chances of survival. The language in which scientific results are presented to the public is likely to influence public attitudes to cancer survival. The popular description of differences in breast cancer survival between regions or socioeconomic groups as a lottery [56] is a case in point. It is frequently repeated in mass-circulation newspapers, but it is misleading. Lotteries are fair. A lottery ticket buys the same chance of winning for rich and poor alike, regardless of residence. This inadequate presentation of the facts may lead the public to believe that, as in a lottery - which demands hope before the draw, but fatalism after it - the differences in survival are simply due to chance, and that little or nothing can be done to change them.
Earlier diagnosis and prompt, universal access to optimal treatment should reduce both socioeconomic inequalities and international differences in survival from breast cancer among women in Europe.
References
- Coleman MP, Estève J, Damiecki P, Arslan A, Renard H. Trends in Cancer Incidence and Mortality (IARC Scientific Publications No 121)Lyon: International Agency for Research on Cancer, 1993. [DOI] [PubMed]
- Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713–1727. doi: 10.1016/s0140-6736(96)90806-5. [DOI] [PubMed] [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52 705 women with breast cancer and 108 411 women without breast cancer. Lancet. 1997;350:1047–1059. [PubMed] [Google Scholar]
- Miller AB, Chamberlain J, Day NE, et al. Report on a workshop of the UICC project on evaluation of screening for cancer. . Int J Cancer. 1990;46:761–769. doi: 10.1002/ijc.2910460502. [DOI] [PubMed] [Google Scholar]
- Chamberlain J, Moss SM, Kirkpatrick AE, et al. National Health Service breast screening programme results for 1991-2. BMJ. 1993;307:353–356. doi: 10.1136/bmj.307.6900.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coebergh JWW, van der Heijden LH, Janssen-Heijnen MLG. Cancer Incidence and Survival in the Southeast of The Netherlands 1955-1994 Eindhoven:IKZ. 1995.
- Andreasen AH, Andersen KW, Madsen M, et al. Regional trends in breast cancer incidence and mortality in Denmark prior to mammographic screening. Br J Cancer. 1994;70:133–137. doi: 10.1038/bjc.1994.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaridze DG, Basieva TH. Incidence of cancer of the lung, stomach, breast, and cervix in the USSR: pattern and trends. Cancer Caus Control. 1990;1:39–49. doi: 10.1007/BF00053182. [DOI] [PubMed] [Google Scholar]
- Ranstam J, Janzon L, Olsson H. Rising incidence of breast cancer among young women in Sweden. Br J Cancer. 1990;61:120–122. doi: 10.1038/bjc.1990.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewertz M, Carstensen B. Trends in breast cancer incidence and mortality in Denmark, 1943-1982. Int J Cancer. 1988;41:46–51. doi: 10.1002/ijc.2910410110. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Estève J. Trends in cancer incidence and mortality in the United Kingdom. Cancer Statistics: Registrations of Cancer Diagnosed in England and Wales. Series MB1 no. 22. London: HMSO, 1989:8–13. [Google Scholar]
- Early Breast Cancer Trialists' Collaborative Group Systemic treatment of early breast cancer by hormonal, cytotoxic or immune therapy. . Lancet. 1992;339:1-15–71-85. [PubMed] [Google Scholar]
- Sant M, Capocaccia R, Verdecchia A, et al. Survival of women with breast cancer in Europe: variation with age, year of diagnosis and country. Int J Cancer. 1998;77:679–683. doi: 10.1002/(SICI)1097-0215(19980831)77:5<679::AID-IJC3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Babb P, Damiecki P, et al. Cancer Survival Trends in England and Wales 1971-1995: Deprivation and NHS RegionSeries SMPS no 61 London: The Stationary Office. 1999.
- Hermon C, Beral V. Breast cancer mortality rates are levelling off or beginning to decline in many western countries: analysis of time trends, age-cohort and age-period models of breast cancer mortality in 20 countries. Br J Cancer. 1996;73:955–960. doi: 10.1038/bjc.1996.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrino F, Sant M, Verdecchia A, (eds):, et al. Survival of Cancer Patients in Europe: the EUROCARE Study (IARC Scientific Publications No 132) Lyon: International Agency for Research on Cancer. 1995.
- Berrino F, Capocaccia R, Estève J, (eds):, et al. Survival of Cancer Patients in Europe: the EUROCARE Study, II (IARC Scientific Publications No 151) Lyon: International Agency for Research on Cancer. 1999.
- Quinn MJ, Martinez-Garcia C, Berrino F, et al. Variations in survival from breast cancer in Europe by age and country, 1978-1989. Eur J Cancer. 1998;34:2204–2211. doi: 10.1016/s0959-8049(98)00323-2. [DOI] [PubMed] [Google Scholar]
- Coebergh JWW, Sant M, Berrino F, (eds):, et al. Survival of adult cancer patients in Europe diagnosed from 1978-89: the EURO-CARE II study. Special issue. Eur J Cancer. 1998;34:2137–2278. [Google Scholar]
- Berrino F, Gatta G, Chessa F, et al. Introduction: the EUROCARE II study. Eur J Cancer. 1998;34:2139–2153. doi: 10.1016/s0959-8049(98)00334-7. [DOI] [PubMed] [Google Scholar]
- Anon Clinical trials and clinical practice. Lancet. 1998;342:877–878. [PubMed] [Google Scholar]
- Fentiman IS, Tirelli U, Monfardini S, et al. Cancer in the elderly: why so badly treated? . Lancet. 1990;335:1020–1022. doi: 10.1016/0140-6736(90)91075-l. [DOI] [PubMed] [Google Scholar]
- Harries SA, Lawrence RN, Scrivener R, et al. A survey of the management of breast cancer in England and Wales. Ann R Coll Surg Engl. 1996;78:197–202. [PMC free article] [PubMed] [Google Scholar]
- Richards MA, Wolfe C, Tilling K, et al. Variations in the management and survival of women under 50 years with breast cancer in the South East Thames Region. Br J Cancer. 1996;73:751–757. doi: 10.1038/bjc.1996.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb PA, Carbone PP. Cancer treatment and age: patient perspectives. J Natl Cancer Inst. 1993;85:1580–1584. doi: 10.1093/jnci/85.19.1580. [DOI] [PubMed] [Google Scholar]
- Selby P, Gillis C, Haward R. Benefits from specialised cancer care. Lancet. 1996;348:313–318. doi: 10.1016/s0140-6736(96)02482-8. [DOI] [PubMed] [Google Scholar]
- Estève J, Benhamou E, Croasdale M, et al. Relative survival and the estimation of net survival: elements for further discussion. Stat Med. 1990;9:529–538. doi: 10.1002/sim.4780090506. [DOI] [PubMed] [Google Scholar]
- Cancer Research Campaign Trends in Cancer Survival in Great Britain: Cases Registered between 1960 and 1974 London: CRC. 1982.
- Silman AJ, Evans SJ. Regional differences in survival from cancer. Comm Med. 1981;3:291–297. [PubMed] [Google Scholar]
- Chouillet AM, Bell CMJ, Hiscox JG. Management of breast cancer in southeast England. BMJ. 1994;308:168–171. doi: 10.1136/bmj.308.6922.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod U, Twelves CJ, Ross S, et al. A comparison of the care received by women with breast cancer living in affluent and deprived areas. Br J Cancer. 1998;78(suppl):S15. [Google Scholar]
- All-Party Parliamentary Group on Breast Cancer Improving Outcomes in Breast Cancer London: House of Commons. 1998.
- Twelves CJ, Thomson CS, Gould A, et al. Variation in the survival of women with breast cancer in Scotland. Br J Cancer . 1998;78:566–571. doi: 10.1038/bjc.1998.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis CR, Hole DJ. Survival outcome of care by specialist surgeons in breast cancer: a study of 3786 patients in the west of Scotland. BMJ. 1996;312:145–148. doi: 10.1136/bmj.312.7024.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrino F, Estève J, Coleman MP. Basic issues in the estimation and comparison of cancer patient survival. Survival of Cancer Patients in Europe: the EUROCARE Study. (IARC Scientific Publications No. 132) Berrino F, Sant M, Verdecchia A, et al (eds). Lyon: International Agency for Research on Cancer. 1995. pp. 1–14.
- Kogevinas M, Marmot MG, Fox AJ, et al. Socioeconomic differences in cancer survival. J Epidemiol Comm Health. 1991;45:216–219. doi: 10.1136/jech.45.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp L, Finlayson AR, Black RJ. Cancer survival and deprivation in Scotland. J Epidemiol Comm Health . 1995;49(suppl):S79. [Google Scholar]
- Carnon AG, Ssemwogerere A, Lamont DW, et al. Relation between socioeconomic deprivation and pathological prognostic factors in women with breast cancer. BMJ. 1994;309:1054–1057. doi: 10.1136/bmj.309.6961.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers CTM, Mackenbach J, Lutz J-M, et al. Deprivation and survival from breast cancer. Br J Cancer . 1995;72:738–743. doi: 10.1038/bjc.1995.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MA, Smith P, Ramirez AJ, et al. The influence on survival of delay in the presentation and treatment of symptomatic breast cancer. Br J Cancer. 1999;79:858–864. doi: 10.1038/sj.bjc.6690137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon: stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312:1604–1608. doi: 10.1056/NEJM198506203122504. [DOI] [PubMed] [Google Scholar]
- McCarthy M, Bore J. Treatment of breast cancer in two teaching hospitals: a comparison with consensus guidelines. Eur J Cancer. 1991;27:579–582. doi: 10.1016/0277-5379(91)90222-y. [DOI] [PubMed] [Google Scholar]
- Royal College of Radiologists Medical Manpower and Workload in Clinical Oncology in the United Kingdom London: Royal College of Radiologists. 1991. [DOI] [PubMed]
- Sainsbury R, Haward R, Rider L, et al. Influence of clinician workload and patterns of treatment on survival from breast cancer. Lancet. 1995;345:1265–1270. doi: 10.1016/s0140-6736(95)90924-9. [DOI] [PubMed] [Google Scholar]
- Royal College of Radiologists A National Audit of Waiting Times for Radiotherapy London: Royal College of Radiologists. 1998.
- Expert Advisory Group on Cancer A Policy Framework for Commissioning Cancer Services London: Department of Health. 1995.
- Engeland A, Haldorsen T, Dickman PW, et al. Relative survival of cancer patients: a comparison between Denmark and other Nordic countries. Acta Oncol. 1998;37:49–59. doi: 10.1080/028418698423177. [DOI] [PubMed] [Google Scholar]
- Ravi S. But can we rely on the statistics? [e-letter]. . BMJ. 1999;318:1163. [Google Scholar]
- Heasman MA, Lipworth L. Accuracy of Certification of Cause of Death Studies on Medical and Population Subjects No 20 London: HMSO. 1966.
- Alderson MR, Meade TW. Accuracy of diagnosis on death certificates compared with that in hospital records. Br J Prev Soc Med. 1967;21:22–29. doi: 10.1136/jech.21.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy CL, Muir CS. The international comparability of cancer mortality data: results of an international death certificate study. . Am J Epidemiol. 1989;129:934–946. doi: 10.1093/oxfordjournals.aje.a115226. [DOI] [PubMed] [Google Scholar]
- Ashworth TG. Inadequacy of death certification: proposal for change. J Clin Pathol. 1991;44:265–268. doi: 10.1136/jcp.44.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ederer F, Axtell LM, Cutler SJ. The relative survival: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- Estève J, Benhamou E, Raymond L. Statistical Methods in Cancer Research, Volume IV Descriptive Epidemiology (IARC Scientific Publications No 128) Lyon: International Agency for Research on Cancer. 1994. [PubMed]
- Department of Health Saving Lives: our Healthier Nation London: Department of Health. 1999.
- Sikora K. Breast cancer: why Britain's women deserve better. Reader's Digest. 1994;145(November):55–61. [Google Scholar]


