Abstract
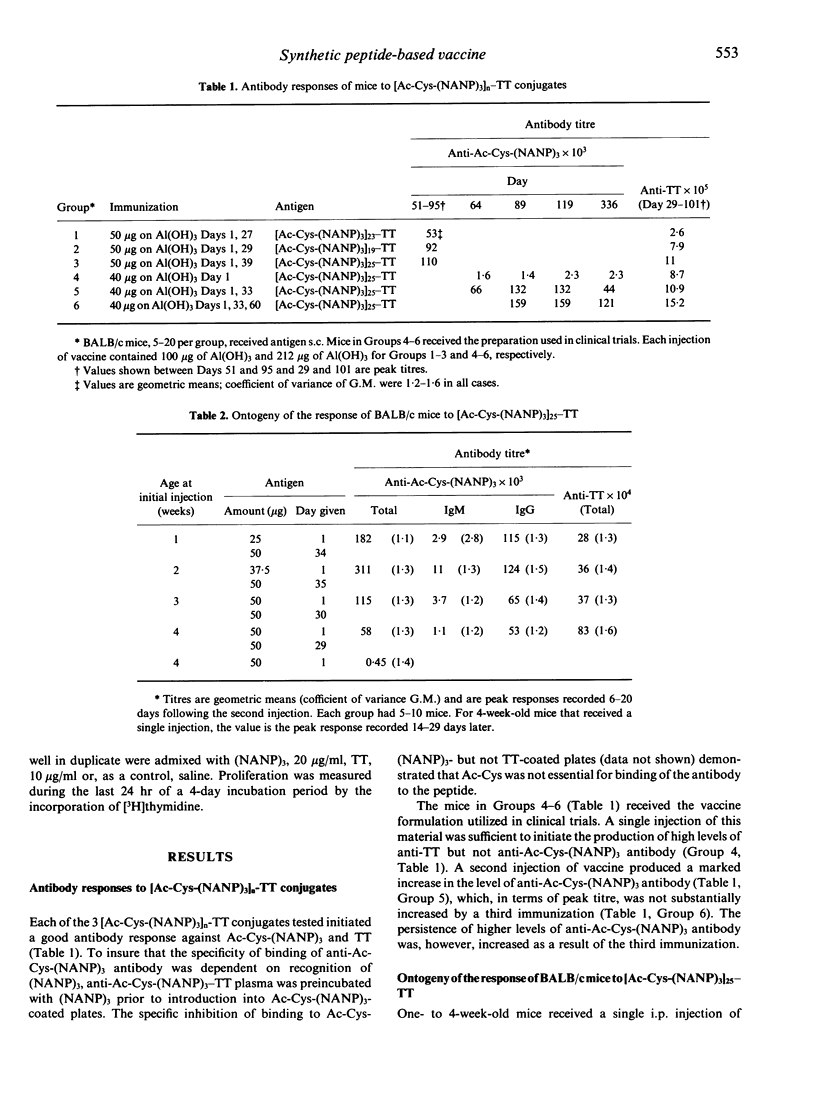
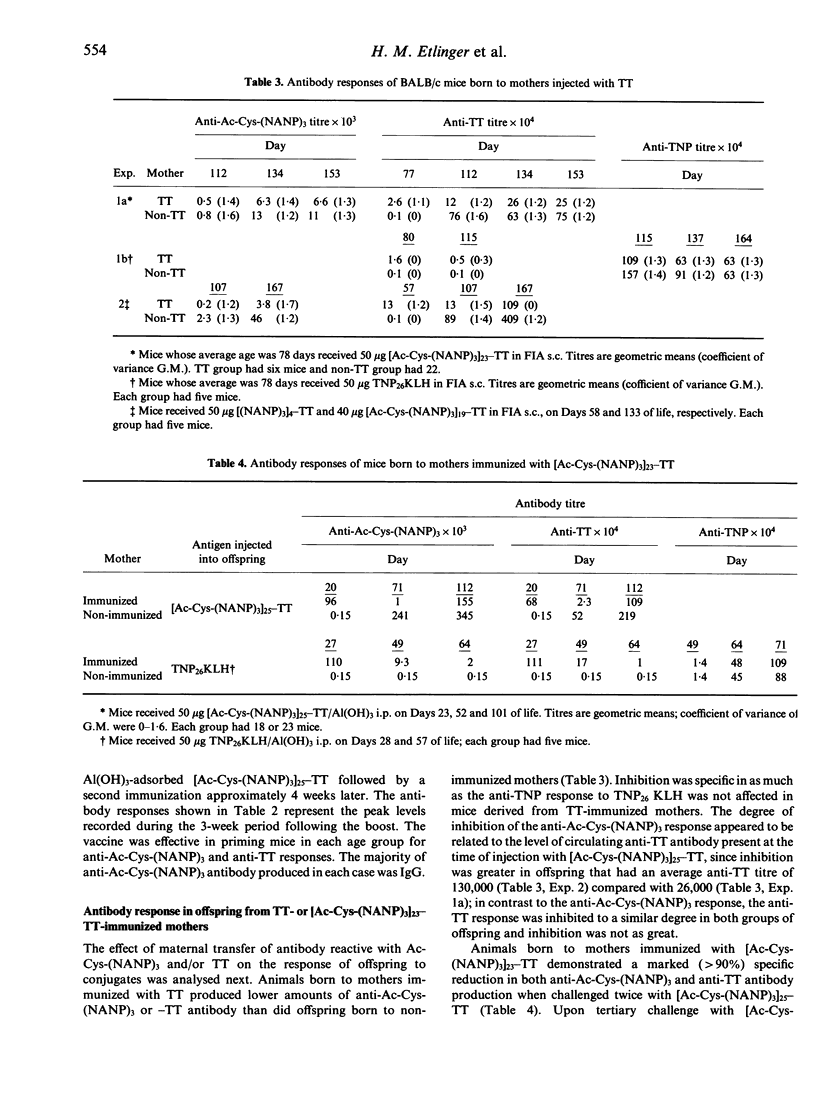
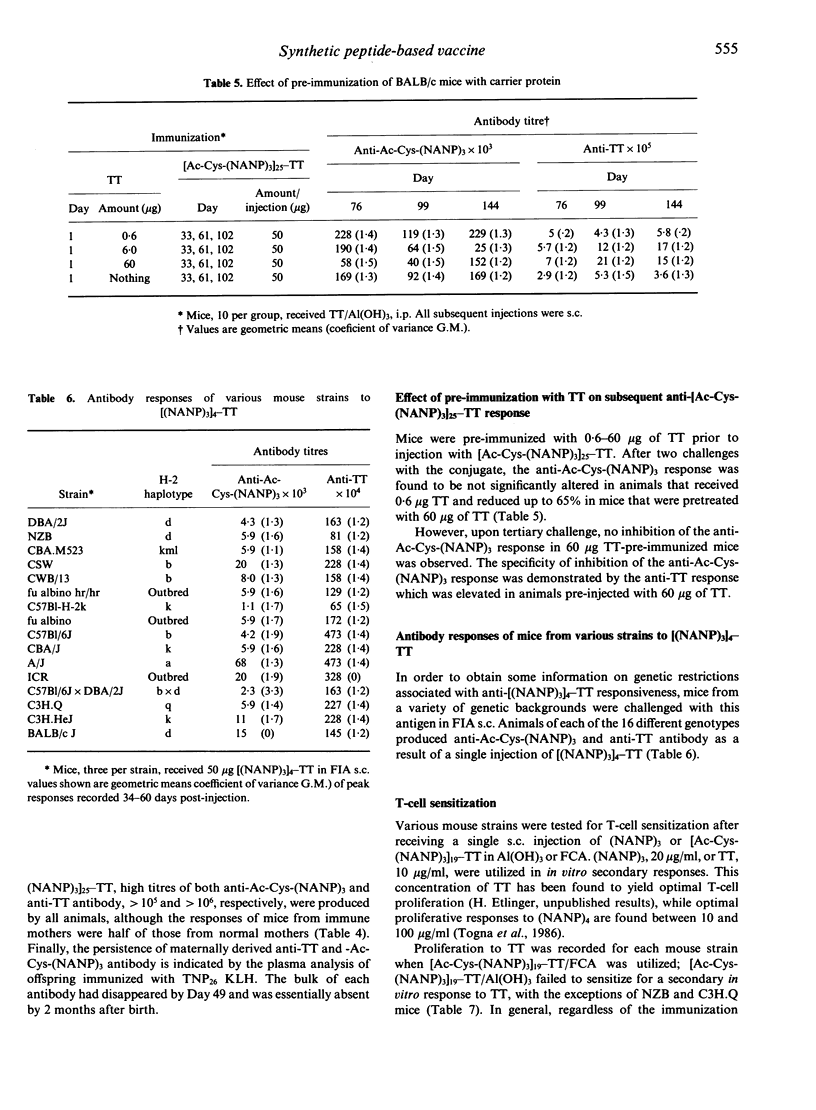
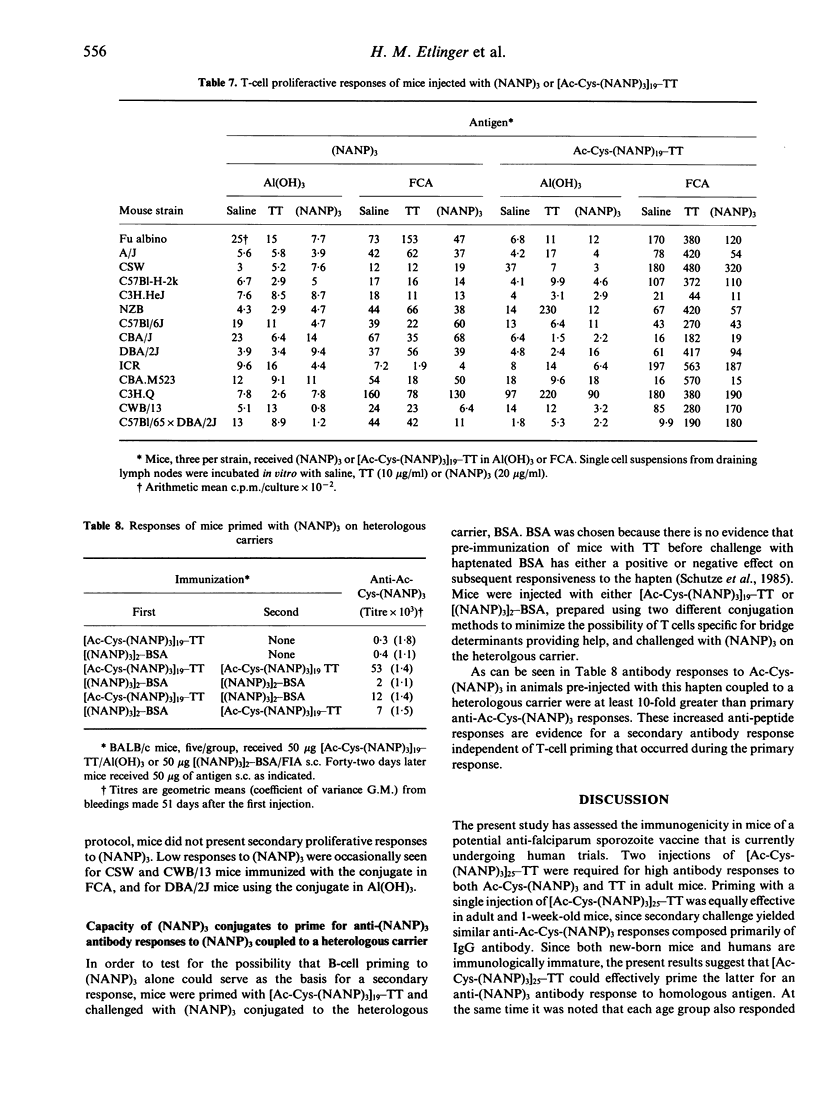
The anti-P. falciparum sporozoite vaccine consisting of the synthetic peptide, Ac-Cys-(NANP)3, conjugated to the protein tetanus toxoid (TT), [Ac-Cys-(NANP)3]25-TT, is currently undergoing human trials. The purpose of the present study was to assess various immunological parameters of this vaccine in mice, which have practical implications in humans. Two injections of [Ac-Cys-(NANP)3]25-TT adsorbed to Al(OH)3 were required to elicit a high antibody response against both Ac-Cys-(NANP)3 and TT. The vaccine initiated equivalent Ac-Cys-(NANP)3 priming for a secondary IgG response in 1-week-old and adult mice. Immunization of female mice with TT or [Ac-Cys-(NANP)3]23-TT prior to mating resulted in offspring that passively received anti-Ac-Cys-(NANP)3 and/or anti-TT antibody and that had reduced secondary responses to Ac-Cys-(NANP)3 and TT. Tertiary challenge with vaccine could substantially overcome such inhibition. Preimmunization of adult mice with TT resulted in a specific inhibition of the anti-Ac-Cys-(NANP)3 antibody response that disappeared following tertiary challenge with the vaccine. The conjugate initiated an antibody response against Ac-Cys-(NANP)3 and TT in mice of 16 different genotypes; only very low T-cell proliferative responses to (NANP)3 were observed for some of these strains. Mice injected with (NANP)3 coupled to protein demonstrated a secondary response to Ac-Cys-(NANP)3 when challenged with (NANP)3 on a heterologous carrier, indicating that B-cell priming alone may be sufficient for a secondary antibody response. These results demonstrate that the vaccine has favourable and unfavourable characteristics in mice; the potential for both exists in humans.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou W. R., Hoffman S. L., Sherwood J. A., Hollingdale M. R., Neva F. A., Hockmeyer W. T., Gordon D. M., Schneider I., Wirtz R. A., Young J. F. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987 Jun 6;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Ballou W. R., Rothbard J., Wirtz R. A., Gordon D. M., Williams J. S., Gore R. W., Schneider I., Hollingdale M. R., Beaudoin R. L., Maloy W. L. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science. 1985 May 24;228(4702):996–999. doi: 10.1126/science.2988126. [DOI] [PubMed] [Google Scholar]
- Briand J. P., Muller S., Van Regenmortel M. H. Synthetic peptides as antigens: pitfalls of conjugation methods. J Immunol Methods. 1985 Apr 8;78(1):59–69. doi: 10.1016/0022-1759(85)90329-1. [DOI] [PubMed] [Google Scholar]
- Chen D. H., Tigelaar R. E., Weinbaum F. I. Immunity to sporozoite-induced malaria infeciton in mice. I. The effect of immunization of T and B cell-deficient mice. J Immunol. 1977 Apr;118(4):1322–1327. [PubMed] [Google Scholar]
- Choromanski L., Kuhn R. E. Repeated antigenic stimulation overcomes immunosuppression in experimental Chagas' disease. Immunology. 1986 Oct;59(2):289–294. [PMC free article] [PubMed] [Google Scholar]
- Corradin G., Etlinger H. M., Chiller J. M. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977 Sep;119(3):1048–1053. [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Del Giudice G., Cooper J. A., Merino J., Verdini A. S., Pessi A., Togna A. R., Engers H. D., Corradin G., Lambert P. H. The antibody response in mice to carrier-free synthetic polymers of Plasmodium falciparum circumsporozoite repetitive epitope is I-Ab-restricted: possible implications for malaria vaccines. J Immunol. 1986 Nov 1;137(9):2952–2955. [PubMed] [Google Scholar]
- Egan J. E., Weber J. L., Ballou W. R., Hollingdale M. R., Majarian W. R., Gordon D. M., Maloy W. L., Hoffman S. L., Wirtz R. A., Schneider I. Efficacy of murine malaria sporozoite vaccines: implications for human vaccine development. Science. 1987 Apr 24;236(4800):453–456. doi: 10.1126/science.3551073. [DOI] [PubMed] [Google Scholar]
- Etlinger H. M., Felix A. M., Gillessen D., Heimer E. P., Just M., Pink J. R., Sinigaglia F., Stürchler D., Takacs B., Trzeciak A. Assessment in humans of a synthetic peptide-based vaccine against the sporozoite stage of the human malaria parasite, Plasmodium falciparum. J Immunol. 1988 Jan 15;140(2):626–633. [PubMed] [Google Scholar]
- Ferreira A., Schofield L., Enea V., Schellekens H., van der Meide P., Collins W. E., Nussenzweig R. S., Nussenzweig V. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science. 1986 May 16;232(4752):881–884. doi: 10.1126/science.3085218. [DOI] [PubMed] [Google Scholar]
- Good M. F., Berzofsky J. A., Maloy W. L., Hayashi Y., Fujii N., Hockmeyer W. T., Miller L. H. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986 Aug 1;164(2):655–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysin J., Barnwell J., Schlesinger D. H., Nussenzweig V., Nussenzweig R. S. Neutralization of the infectivity of sporozoites of Plasmodium knowlesi by antibodies to a synthetic peptide. J Exp Med. 1984 Sep 1;160(3):935–940. doi: 10.1084/jem.160.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington D. A., Clyde D. F., Losonsky G., Cortesia M., Murphy J. R., Davis J., Baqar S., Felix A. M., Heimer E. P., Gillessen D. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987 Jul 16;328(6127):257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Tokuhisa T. Epitope-specific regulation. I. Carrier-specific induction of suppression for IgG anti-hapten antibody responses. J Exp Med. 1982 Jun 1;155(6):1730–1740. doi: 10.1084/jem.155.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Tokuhisa T., Parks D. R., Herzenberg L. A. Epitope-specific regulation. II. A bistable, Igh-restricted regulatory mechanism central to immunologic memory. J Exp Med. 1982 Jun 1;155(6):1741–1753. doi: 10.1084/jem.155.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel R. A., Anderson P. W. Oligosaccharide-protein conjugate vaccines induce and prime for oligoclonal IgG antibody responses to the Haemophilus influenzae b capsular polysaccharide in human infants. J Exp Med. 1986 Feb 1;163(2):262–269. doi: 10.1084/jem.163.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C. O., Arnon R., Sela M. Effect of carrier on the immunogenic capacity of synthetic cholera vaccine. Mol Immunol. 1985 Dec;22(12):1333–1339. doi: 10.1016/0161-5890(85)90054-9. [DOI] [PubMed] [Google Scholar]
- Keller O., Rudinger J. Preparation and some properties of maleimido acids and maleoyl derivatives of peptides. Helv Chim Acta. 1975 Mar 12;58(2):531–541. doi: 10.1002/hlca.19750580224. [DOI] [PubMed] [Google Scholar]
- Mazier D., Mellouk S., Beaudoin R. L., Texier B., Druilhe P., Hockmeyer W., Trosper J., Paul C., Charoenvit Y., Young J. Effect of antibodies to recombinant and synthetic peptides on P. falciparum sporozoites in vitro. Science. 1986 Jan 10;231(4734):156–159. doi: 10.1126/science.3510455. [DOI] [PubMed] [Google Scholar]
- Mitchison N. A. The carrier effect in the secondary response to hapten-protein conjugates. I. Measurement of the effect with transferred cells and objections to the local environment hypothesis. Eur J Immunol. 1971 Jan;1(1):10–17. doi: 10.1002/eji.1830010103. [DOI] [PubMed] [Google Scholar]
- Nardin E. H., Nussenzweig R. S., Bryan J. H., McGregor I. A. Congenital transfer of antibodies against malarial sporozoites detected in Gambian infants. Am J Trop Med Hyg. 1981 Nov;30(6):1159–1163. doi: 10.4269/ajtmh.1981.30.1159. [DOI] [PubMed] [Google Scholar]
- Nussenzweig R., Vanderberg J., Most H. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. IV. Dose response, specificity and humoral immunity. Mil Med. 1969 Sep;134(10):1176–1182. [PubMed] [Google Scholar]
- Potocnjak P., Yoshida N., Nussenzweig R. S., Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980 Jun 1;151(6):1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajewsky K., Schirrmacher V., Nase S., Jerne N. K. The requirement of more than one antigenic determinant for immunogenicity. J Exp Med. 1969 Jun 1;129(6):1131–1143. doi: 10.1084/jem.129.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe M. J., Julius M. H. H-2-restricted T-B cell interactions involved in polyspecific B cell responses mediated by soluble antigen. Eur J Immunol. 1982 Aug;12(8):634–641. doi: 10.1002/eji.1830120803. [DOI] [PubMed] [Google Scholar]
- Schutze M. P., Leclerc C., Jolivet M., Audibert F., Chedid L. Carrier-induced epitopic suppression, a major issue for future synthetic vaccines. J Immunol. 1985 Oct;135(4):2319–2322. [PubMed] [Google Scholar]
- Schutze M. P., Leclerc C., Vogel F. R., Chedid L. Epitopic suppression in synthetic vaccine models: analysis of the effector mechanisms. Cell Immunol. 1987 Jan;104(1):79–90. doi: 10.1016/0008-8749(87)90008-6. [DOI] [PubMed] [Google Scholar]
- Togna A. R., Del Giudice G., Verdini A. S., Bonelli F., Pessi A., Engers H. D., Corradin G. Synthetic Plasmodium falciparum circumsporozoite peptides elicit heterogenous L3T4+ T cell proliferative responses in H-2b mice. J Immunol. 1986 Nov 1;137(9):2956–2960. [PubMed] [Google Scholar]
- Young J. F., Hockmeyer W. T., Gross M., Ballou W. R., Wirtz R. A., Trosper J. H., Beaudoin R. L., Hollingdale M. R., Miller L. H., Diggs C. L. Expression of Plasmodium falciparum circumsporozoite proteins in Escherichia coli for potential use in a human malaria vaccine. Science. 1985 May 24;228(4702):958–962. doi: 10.1126/science.2988125. [DOI] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Hollingdale M. R., Cochrane A. H., Quakyi I., Nussenzweig R. S., Nussenzweig V. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985 Jun 21;228(4706):1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]


